7. Background
7.1 History of F-gases – from CFCs to HFOs
Hydrofluoroolefins (HFO) are the fourth generation of synthetic refrigerants, succeeding the generations of natural refrigerants, CFCs, HCFCs and HFCs. This section briefly describes the three earlier generations of fluorinated refrigerants developed and used before HFOs came on the market. This section also briefly explains why CFCs, HCFCs and HFCs have been or are in the process of being phased down or out. The major global agreements that are the driving force behind the phase-outs or phasedowns are described in Chapter 6.
The first generation of refrigerants (1830s–1930s)
Before the introduction of halogenated refrigerants, multiple different substances with good thermodynamic characteristics were used, several of which are still in use today, including ammonia, propane, and carbon dioxide. Many of the first available refrigerants, including ammonia, methyl chloride, and sulphur dioxide, are toxic and/or flammable. When incorrectly handled, severe health and safety risks are associated with their use, and several fatal accidents have occurred.
McLinden, M. O. & Huber, M. L. (2020)
Chlorofluorocarbons (1930s–1990s) and hydrochlorofluorocarbons (1940s–2010s)
CFCs are fully halogenated chlorofluorocarbons; CFCs were introduced in the late 1920s under the popular name of freon. CFCs have several properties that make them especially suitable for various applications: They are non-flammable, non-toxic and inert in the lower atmosphere with an atmospheric lifetime of 100 years.
Elkins (1999)
NOAA (n.d.)
Ozone Secretariat (2018a)
vanLoon & Duffy (2011)
Papst (2020)
Ozone Secretariat (2018a)
Hydrofluorocarbons (1990es–2030)
HFCs were developed to substitute CFCs and HCFCs following the global agreement to phase out these substances due to their impact on the ozone layer. HFCs first entered the market in the 1990s. HFCs are widely used as refrigerants, blowing agents in foams and propellants in aerosol sprays. HFCs do not contain any chlorine and are not ozone-depleting substances (ODS), but HFCs are very potent greenhouse gases with a high GWP. For instance, R-134a, used in mobile air-conditioning systems (MACs), has a GWP of 1,430. The GWP for certain HFCs is as high as 14,800 (HFC-23). Because of their potency as greenhouse gases, it was decided to initiate a global HFC phasedown with the Kigali Amendment to the Montreal Protocol in 2016.
Ozone Secretariat (2018a)
The next primary refrigerant?
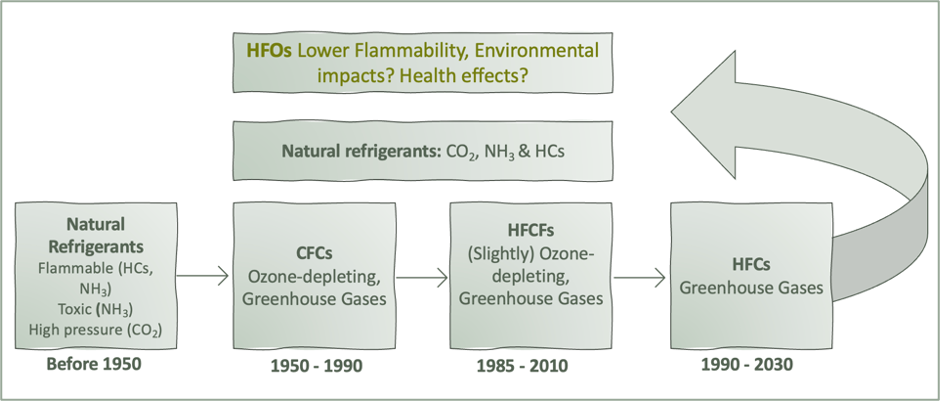
Figure 1 Historical development of refrigerants, adapted from the report ‘Legislation and practices for End-of-life Management of refrigerants and other F-gases in Norway and the EU’ by Asphjell et al. (2023)
There exist multiple alternatives that can replace most HFCs in different appliances, both the so-called natural refrigerants and a new generation of fluorinated refrigerants. No 'one size fits all' solution exists due to the wide array of applications where HFCs are in use and the alternatives' different operating pressures, safety properties, and thermodynamic properties. Alternatives to HFCs include:
- Natural refrigerants
- Hydrofluoroolefins (HFOs)
- HFC-HFO Blends
Many low GWP alternatives present other challenges, environmental, safety and cost implications, many natural refrigerants are flammable (hydrocarbons), and some are toxic (ammonia). Some HFO substances are mildly flammable, and several HFOs break down to TFA in the environment; some partly and others completely (see section 6.6). Many refrigerants will likely be blends of currently used refrigerants.
A3 | B3 | Higher Flammability |
A2 | B2 | Flammable |
A2L | B2L | Lower Flammability |
A1 | B1 | No Flame Propagation |
Lower toxicity | Higher Toxicity |
Figure 2 Refrigerant safety classification ISO 817 (ISO 817:2014)
7.2 Hydrofluoroolefins
Hydrofluoroolefins (HFOs) are, together with hydrochlorofluoroolefins (HCFOs), considered the fourth generation of fluorinated refrigerant gases. Hydrofluoroolefins are unsaturated hydrofluorocarbons composed of hydrogen, fluorine, and carbon. HCFOs contain chlorine as well. The carbon-carbon double bond greatly reduces their atmospheric lifetime. Many HFOs are low-pressure fluids with a high boiling point.
Ozone Secretariat (2018b)
Mota-Babiloni et al (2015)
Box 1: Nomenclature for Hydrofluoroolefins:
Exemplified with 1234yf.
HFO- 1234yf
HFC-1234yf
R1234yf
uHFC- 1234yf
HFOs are not a recently developed novelty. The first synthesis of HFO-1234yf was reported in 1946. But when the EU adopted the MAC directive in 2006, which prohibits refrigerants with a GWP higher than 150 in mobile air-conditioning of new vehicles, HFO-1234yf was found to be a suitable alternative for mobile air-conditioning systems.
McLinden, M. O. & Huber, M. L. (2020)
HFOs are mainly produced in the US, China, Japan, and India. There is no manufacturing of these gases in the EU,
Behringer et al. (2021)
Behringer et al (2021) & A-GAS (n.d.)
Regulation (EU) No. 517/2014
Substance name | Main use | GWP 100 |
HFO-1234yf | Refrigerant (esp. mobile air conditioning) | 0.501 |
HFO-1234ze | Refrigerant, foam blowing agent, aerosol propellant | 1.37 |
HFO-1336mzz | Refrigerant, foam blowing agent | 17.9 |
Table 1 HFOs listed in annex II of the F-gas Regulation 517/2014/EU, GWP values Based on the Sixth Assessment Report adopted by the Intergovernmental Panel on Climate Change.
The World Meteorological Organisation frequently publishes a scientific assessment of ozone depletion. The most recent assessment (2022) concludes that there are no comprehensive global datasets on the production or consumption of HFOs.
Regulation (EU) No. 517/2014
European Commission (2022a)
There are also indications of regional concentration increases of HFOs measured in the environment. European atmospheric observations from the two observatories, Dubendorf and Jungfraujoch, have registered increases in the background concentration of some HFOs. Jungfraujoch documented increases from less than 0.01 ppm in 2016 to annual median levels of 0.10 for HFO-1234yf and 0.14 ppt for HFO-1234ze(E) in 2020.
European Commission (2022a)
7.2.1 HFO/HFC Blends
There are several different substance blends of HFOs and HFCs on the market. These blends are manufactured to lower the flammability of the substance or, in other words, enhance their performance while lowering the GWP of the blend.
The gases often used in blends are:
- HFC refrigerants: R32, R125, R152a and R134a
- HFO refrigerants: R1234yf and R1234ze(E)
- And/or natural refrigerants such as R-290, R-600a and R-744
There is a need for further research since there are some uncertainties concerning these blends. There are studies that investigate the stability of blends to see whether they maintain their expected original compositions when in use or try to establish the most reliable route to recovering these gases, including an ongoing Swedish-funded project.
KTH (n.d.)
Mota-Babiloni et al (2015)
European Commission (n.d.)
Table 2 Common market-available blends of HFCs and HFOs (European commission n.d.a). GWP based on the Fourth Assessment Report adopted by the Intergovernmental Panel on Climate Change.
Substance | GWP | Composition | Safety Group | Replacement for | Suitable for |
R448A | 1387 | R32/125/1234yf/1234ze(E)/134a | A1 | R404A | Centralised systems for commercial refrigeration, condensing units, and refrigerated vehicles |
R449A | 1397 | R32/125 /1234yf/134a | A1 | R404A | Centralised systems for commercial refrigeration, condensing units, industrial refrigeration, refrigerated vehicles |
R452A | 2140 | R32/125/1234yf | A1 | R404A | Refrigerated vehicles, refrigerated containers |
R454C | 148 | R32/1234yf | A2L | R410A | Heat pumps and chillers |
R455A | 148 | R32/1234yf/CO2 | A2L | R404A | Chiller |
R513A | 631 | R1234yf/134a | A1 | R134a | Condensing units, industrial refrigeration, heat pumps, chillers, refrigerated containers |
R515B | 299 | R1234ze/R227ea | R134a, R450A, R513A, R227ea, R124 | Heat pumps, chillers |
7.3 Natural Refrigerants
The use and application of natural refrigerants have come a long way since the early 1900s, and many of the previous challenges, such as flammability, have been addressed by lowering the amounts of refrigerants and optimising system designs, making them safer to use. There are often additional technical training requirements when working with natural refrigerants to ensure safe use and proper handling. Switching from HFCs to natural refrigerants in RACHP applications requires entirely different systems than switching to fluorinated blends that can be used as drop-ins.
The most commonly used natural refrigerants are carbon dioxide (R744), hydrocarbons, ammonia (R717) and dimethyl ether (R-E170).
7.3.1 Carbon dioxide (R744)
CO2 is a non-flammable and non-toxic refrigerant that operates at a higher pressure than other refrigerants, both fluorinated and natural.
The Natural Voice Magazine (2016)
Ozone Secretariat (2018b).
European Commission (2020).
Ozone Secretariat (2022a).
Ozone Secretariat (2018b).
7.3.2 Hydrocarbons
Numerous different hydrocarbons are used as refrigerants and foam-blowing agents. Some of the most common are listed below. Hydrocarbons have similar thermodynamic properties to fluorinated refrigerants, but hydrocarbons are flammable and, therefore, have higher safety requirements.
Ozone Secretariat (2018b).
- Propane (R290) is a classified A3 refrigerant, meaning there are some limitations from product standards and/or building codes.
- Isobutane (R600a) Isobutane is widely used in low-charge hermetically sealed applications such as refrigerators and freezers.Copenhagen School of Marine Engineering and Technology Management (2023)
- Propylene (R1270) is also classified as an A3 refrigerant, so there are some limitations from product standards and/or building codes. Propylene is mainly used in chillers today.
- Pentane (R601), cyclopentane, and isopentane are applied as foam-blowing agents.European Commission (n.d.)
7.3.3 Ammonia (R717)
Ammonia has been used for over a century. Ammonia has great thermodynamic properties but is toxic and flammable in certain conditions, so additional safety measures are required. Ammonia can be used for both cooling and heating. Due to the toxicity of ammonia, it is often used in conjunction with other refrigerants, such as CO2, in cascade systems to make it safer. Ammonia is used in appliances at an industrial scale.
Ozone Secretariat (2018b).
7.3.4 Dimethyl ether (R-E170)
Dimethyl ether (DME) was one of the first refrigerants and was first used in the late 1800s. Today, DME is used as an aerosol propellant, a (co-)blowing agent for foam, and in refrigerant blends. The application of dimethyl ether is projected to increase in the future. DME is both highly flammable and explosive. DME is often more expensive than other low-GWP non-fluorinated refrigerants since it is chemically synthesised.
Ozone Secretariat (2018b); (2018c); (2018d)
7.4 Environmental Concerns
Market stakeholders often highlight in their marketing that HFOs have little to no adverse impacts on the environment. Therefore, the HFOs are promoted as an environmentally friendly alternative to HFCs. This claim is based on the short atmospheric lifetime of HFOs, low GWP, and zero ODP. However, there are concerns about the environmental impact of HFOs, not least in relation to the current increase in usage and the expected future increase. One of the major environmental concerns is the persistence, aqueous mobility and toxicity of HFO breakdown products, especially trifluoroacetic acid. Moreover, even though HFOs are listed as non-toxic (toxicity level A), some HFO feedstock substances are toxic, have a high GWP or are ODSs.
WMO (2022)
Molar yield = The amount of a substance obtained in a chemical reaction expressed in moles (SI unit for amount of substance)
7.4.1 Trifluoroacetic Acid (TFA)
HFOs have an approximate lifetime of days in the atmosphere before being degraded.
Behringer et al (2021)
Miljødirektoratet (2023)
The OECD’s PFAS definition
PFAS are defined as fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it) i.e., with a few noted exceptions, any chemical with a least a perfluorinated methyl group (-CF3) or a perfluorinated methylene group (-CF2-) is a PFAS (OECD, 2021)
TFA is formed when HFOs are emitted into the atmosphere, where they oxidise. It is uncertain to what extent TFA naturally occur in the environment.
WMO (2022)
Joudan et al (2021)
WMO (2022)
German Environment Agency (2021)
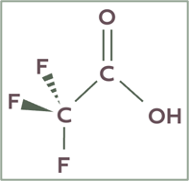
Figure 3 Structure of Trifluoroacetic Acid (TFA)
TFA has a relatively short lifetime in the atmosphere of approximately four months.
Holland et al (2021)
Behringer et al (2021)
Björnsdotter et al (2022)
Brunn et al (2023)
German Environment Agency (2021)
In 2021, the Danish Environmental Protection Agency reported findings of TFA in 219 out of 247 groundwater wells (89% of all samples). TFA was also found in some drinking water supplies; the concentration was lower than 1mg/L for most findings.
Danish Environment Agency (2021)
HFO-1234yf completely breaks down to TFA, and with a continued substitution of HFC-134a with HFO-1234yf, the total amount of TFA deposited from the atmospheric degradation of fluorinated substances is projected to increase by more than 300% in 2050 (compared to 2018). This results in a projected annual increase of 49,718 tonnes of TFA by 2050 from EU-28; 96% will come from the atmospheric degradation of HFO-1234yf.
Behringer et al (2021)
Increasing TFA concentrations also pose a health concern, especially since TFA is persistent and accumulates in water bodies. According to current knowledge, toxicological and ecotoxicological effects are only observed at very high concentrations. However, according to the background report ‘Reducing the input of chemicals into waters: trifluoroacetate (TFA) as a persistent and mobile substance with many sources’ from the German Environmental Agency in 2021, the long-term impacts of TFA are still very uncertain.
German Environment Agency (2021)
Umweltbundesamt (2021)
Atmosphere (2022)
Drikkevandsbekendtgørelsen (2021)
Molar yield of TFA from different HFOs:
1234yf 100%
1234ze(E) <10%
1336mzz(Z) <20%
1225ye(E) 100%
1225ye(Z) 100%
(Behringer et al 2021)
TFA is already widely present in the environment and in freshwater reservoirs. There is a need for further research to better understand the atmospheric and hydrospheric cycle of TFA and to clarify some of the current uncertainties concerning the lifecycle of TFA as well as long-term impacts.
7.4.2 HFC-23
HFC-23 (Trifluoromethane (CHCl3)) is a potent GHG with a GWP100 of 12400.
Myhre et al (2013)
WMO (2022)
Although this study focuses on the end-of-life treatments of HFOs, it is important to note some of the environmental concerns related to the HFO production feedstock since they pose a potential risk for counteracting results obtained due to the Montreal Protocol and the Kigali Amendment, as well as EU strategies.
7.5 Health and Safety
When exposed to high temperatures or high doses of UV light combined with heat, HFOs like HFCs will decompose to toxic substances, including hydrofluoric acids and carbonyl fluoride, raising concerns over potential toxicity hazards in the workplace. In high concentrations, HFOs are asphyxiant, and contact with evaporating liquid can lead to frostbite. Therefore, proper education of practitioners and adequate safety measures in the workplace are crucial. According to a Norwegian study from 2017, there is a lack of publicly available information on HFOs' effect on health.
Ozone Secretariat (2018b)
Fleet et al (2017)
Since the MAC Directive was adopted in 2006, the use of HFO-1234yf as a replacement for HFC-134a in MAC systems has raised some debate that continued up through the 2010s due to safety concerns. Consequently, numerous tests were conducted in the same period.
European Commission (2014b)
BAM (2010)
European Commission (2014a)
HFO foam-blowing agents have similar toxicity exposure limits to HFCs, and exposure concerns include frostbite and oxygen deprivation when large amounts are released in an enclosed space. Several studies have been conducted to determine when foam-blowing agents have degassed sufficiently and when it is safe to re-enter the area in question.
Ozone Secretariat (2023)