3. Results and future perspectives
In this section we show how the AK reduced phosphate fluxes and affected other important sediment processes. The applicability and steps toward a trial in the field are discussed. The data are presented in the text as the mean value of five treatment replicates ± standard deviation, unless otherwise is stated.
Sediment parameters and lab observations
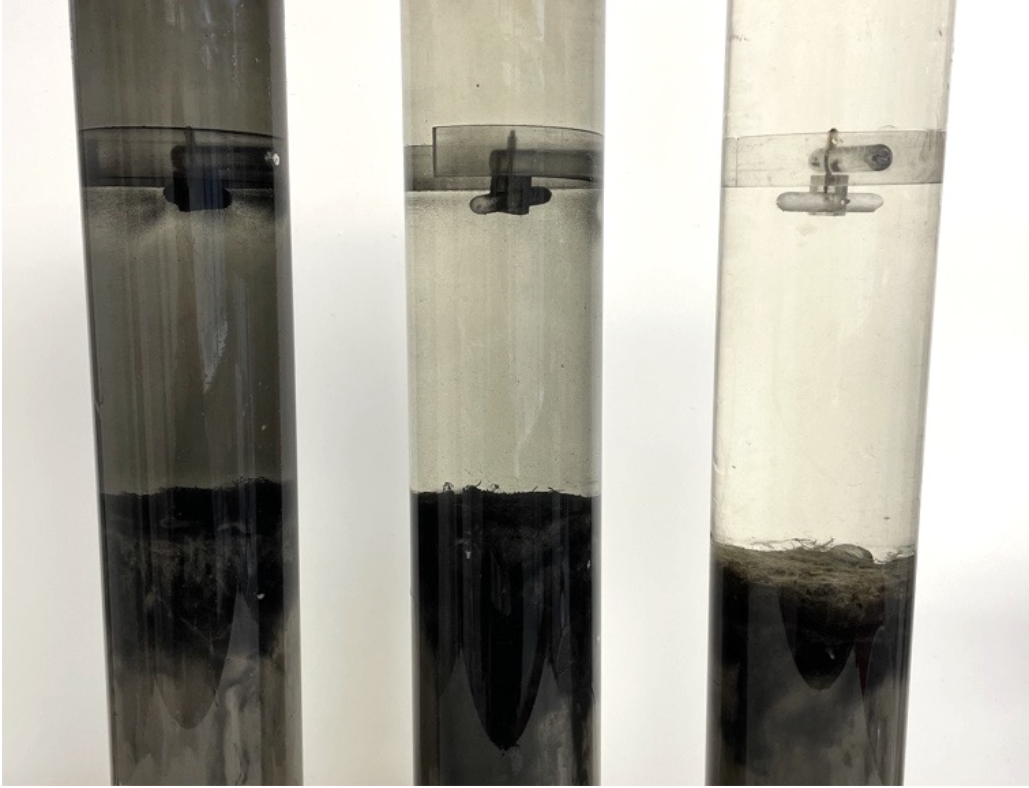
Figure 4. One core per treatment (from left: CTRL, AK300, and AK600) showing varying iron sulfide formation as black precipitation. Notice the precipitation on the core liner walls, magnetic stirrer at the top, and the color of the sediment surface.
In untreated control, i.e., natural conditions in the field, the top 2 cm sediment had a water content of 80 ± 0.01 % and porosity (φ) of 0.9 ± 0.01. Organic matter content was 17 ± 1% dry weight, which is normal for eutrophicated accumulation bottoms, and coherent with existing data collected at Storfjärden (Naturvatten i Roslagen AB 2023). When slicing the sediment (cutting and obtaining different depth segments) at the experiment termination it was clear that most of the solid AK had dissolved, although a few particles of around 5 mm had allocated as deep as 2 cm down into the sediment.
There was a clear visual difference between CTRL and AK cores one month after treatment; the sediment surface, magnetic stirrer and core liner were colored black in the CTRL treatment. In the treatment with activated limestone of low dose (AK300), there was some precipitation but not as pronounced as in CTRL. In AK600, the sediment surface was still brightly colored and very little precipitation had formed (Figure 4). The black precipitate was likely iron sulfide formation, which is limited by iron availability. This interesting observation motivated sampling of the water column for sulfide at the experiment termination, since it was hypothesized that lower iron sulfide formation in AK treatments could lead to release of free sulfide, which it did, see below for more details. As mentioned earlier, iron sulfide is known to decrease the ability of the sediment to bind PO43- which can then be released (Smolders et al. 2006). This effect was detected in untreated CTRL, see below for more details. These observations are typical for anoxic sediments.
Nutrient release: phosphate and ammonium
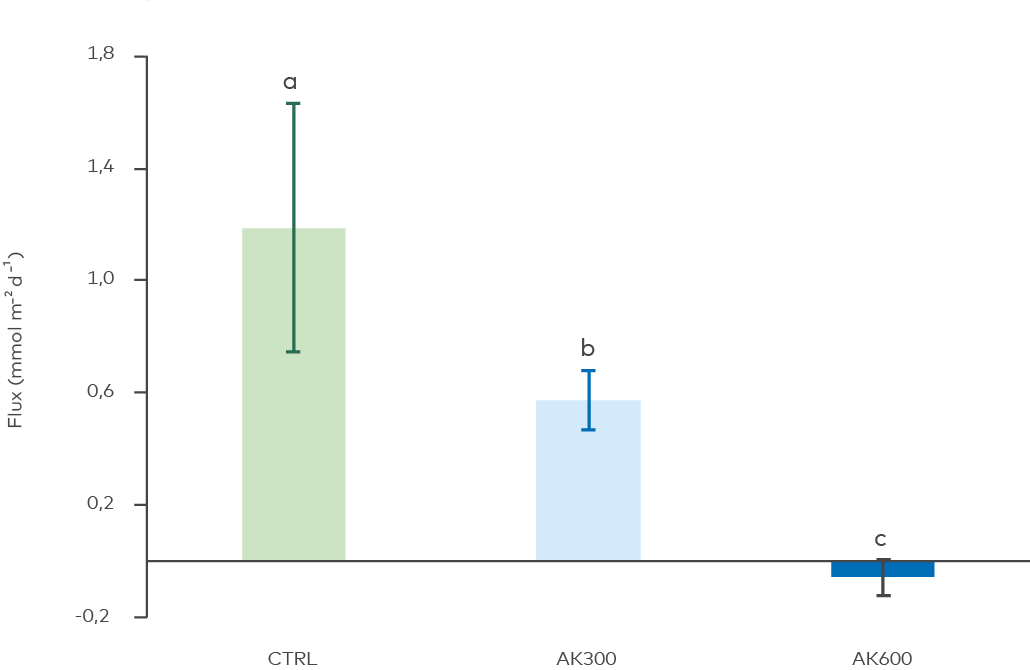
Figure 5. Effects of the treatments on sediment-to-water flux (release of free phosphate per m2 sediment per day). CTRL = untreated sediment; AK300 and AK600 = 300 and 600 g AK/m2. Bars show treatment mean and standard deviation. Different alphabetic letters above the bars denote statistically significant differences between treatments (Kruskal-Wallis, p = 0.002, pairwise Wilcoxon test), i.e., all treatment means differed significantly.
Activated limestone treatment decreased the phosphate release from the sediment, measured as release per m2 over time (flux, Figure 5). The lower dose (300 g/m2) decreased flux by 48%, compared with untreated CTRL. The double dose (600 g/m2) decreased phosphate release by 100%, i.e., stopped it completely. In summary, the AK material was found to successfully decrease the amount of phosphate released from the sediment.
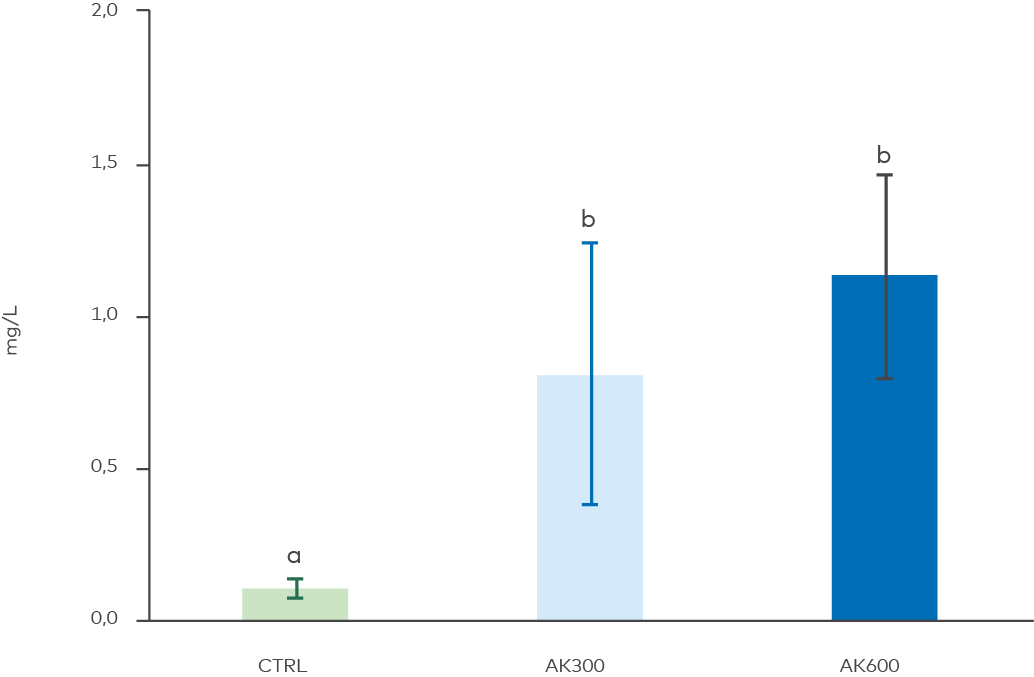
Figure 6. Phosphate concentration in sediment porewater after the experiment termination. Different letters above the bars denote statistically significant differences between treatments (ANOVA, p = 0.003, Tukey’s post-hoc test). AK treatments differed from CTRL. n = 4 for AK600.
Simultaneously, sediment porewater phosphate in the top 2 cm increased in the limestone amended sediment (Figure 6). This effect can likely be attributed to the unrealistic changes in pH caused by the AK (see results below); increased alkalinity has been shown to speed up biological degradation of organic matter, which will generate more dissolved phosphate. Generated bicarbonates by AK can also compete with the phosphate anion for binding sites (Smolders et al. 2006). In any case, this did not increase the flux of phosphate from the sediment to the water, which verifies that the dissolved AK in the superficial sediment layer actively sorbed phosphate through the experiment.
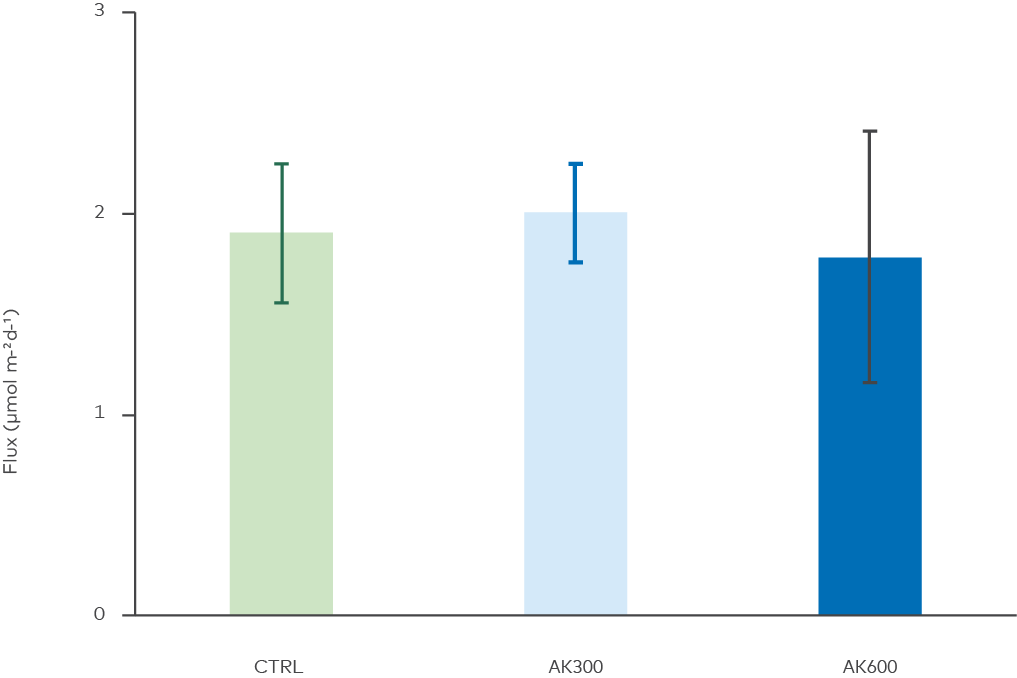
Figure 7. Effects of the treatments on sediment-to-water flux of ammonium. No statistical differences between treatments were found (ANOVA, p = 0.78).
Ammonium is a dissolved and bioavailable form of nitrogen and a nutrient that together with phosphate drives eutrophication in the Baltic Sea. Ammonium fluxes were measured as an estimation of microbial degradation of organic nitrogen such as proteins and nucleic acids. Figure 7 shows that no effect of the AK was detected regarding release of ammonium.
pH
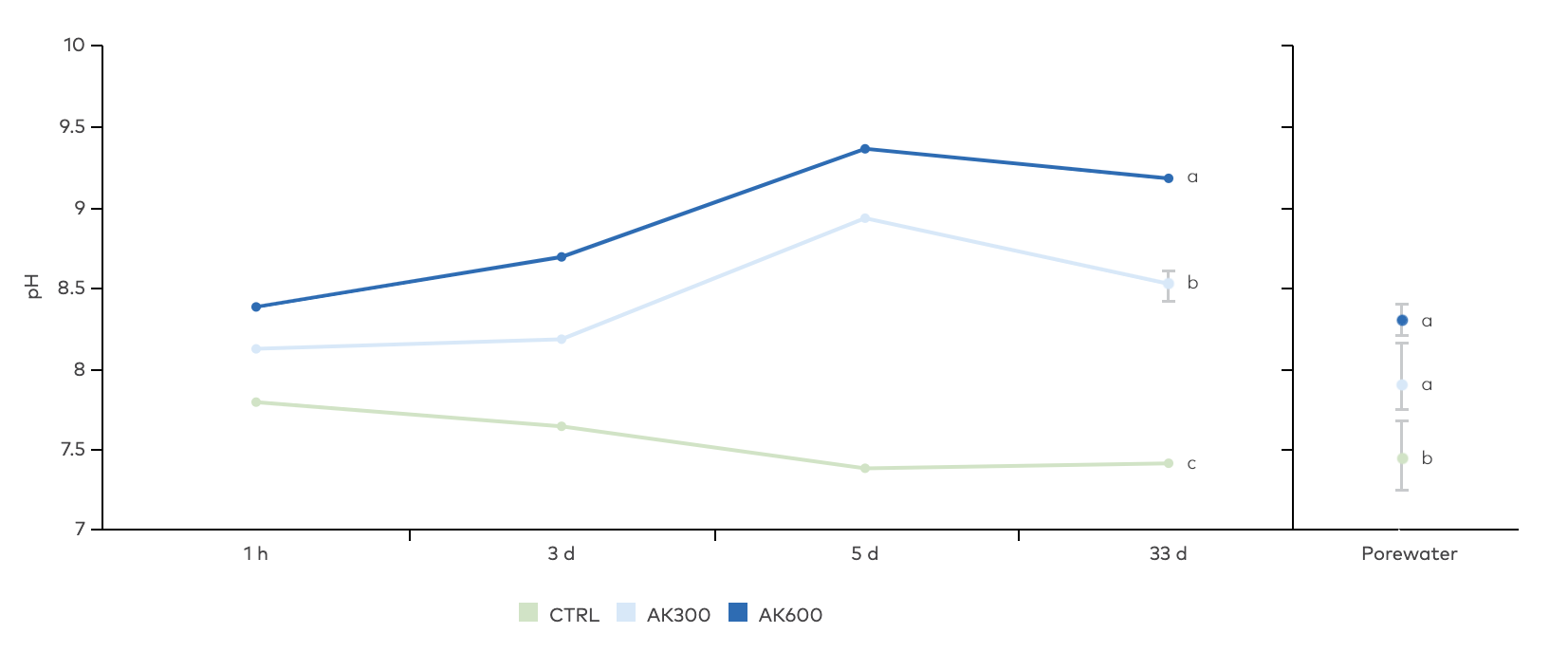
Figure 8. Effects of the treatments on pH in the water overlying the sediment over the first 33 days (left side), and pH in sediment porewater at the experiment termination (right side). Vertical bars show standard deviation of mean. Different letters by the bars indicate significant difference between the groups. pH was found the be statistically different in the water between all treatments at every measurement (ANOVA, p < 0.05, Tukey’s post-hoc test), while pH in the sediment porewater was different in the CTRL compared to the AK treatments. n = 4 for AK600 porewater pH.
pH in the water column above untreated sediment (CTRL) decreased from 7,8 ± 0.005 at the experiment start to 7.3 ± 0.04 at day 33. (Figure 8). In contrast, the alkaline AK increased the pH of both the overlying water and sediment porewater. In the highest dose of 600 g m-2, pH was 9.2 ± 0.04 after 33 days. pH in the treatment with 300 g m-2 AK was also affected but not to the same degree as in the higher dose. Porewater pH was measured at the experiment termination and was 7.5 ± 0.25 in CTRL and increased to 8.0 ± 0.22 and 8.2 ± 0.11 in AK300 and AK600.
pH increase in the AK treatments was expected, since the limestone is an alkaline material, releasing hydroxide ions as it dissolves. It is likely that this increase generated more alkalinity, since phosphate and sulfide, which were elevated (more details below), can contribute to porewater alkalinity in anoxic sediments (Lukawska-Matuszewska 2016).
It also must be considered that the effect of AK on pH was unnaturally amplified in this experimental setup, where buffering capacity was limited in the 1.3 L core water column, that was not replaced during the experiment. Buffering capacity in the field is many orders of magnitude higher and it is therefore improbable that this pH increase would occur in a field situation. This claim is supported by monitoring data collected in Kyrkviken bay that has been treated with 300 g AK/m2 without any effect on pH in the bottom water (Björkman, 2023). It is important to point out that several of the effects measured in this study can be linked to changes in pH, since pH is a primary controller that can alter chemical speciation and energy yields of compounds of microbial metabolism. For example, the energy yield for fermentation of, e.g., acetate and lactate in microbial sulfate reduction is at its lowest at neutral pH and increases considerably as pH either increases or decreases (Jin and Kirk 2018).
Hydrogen sulfide
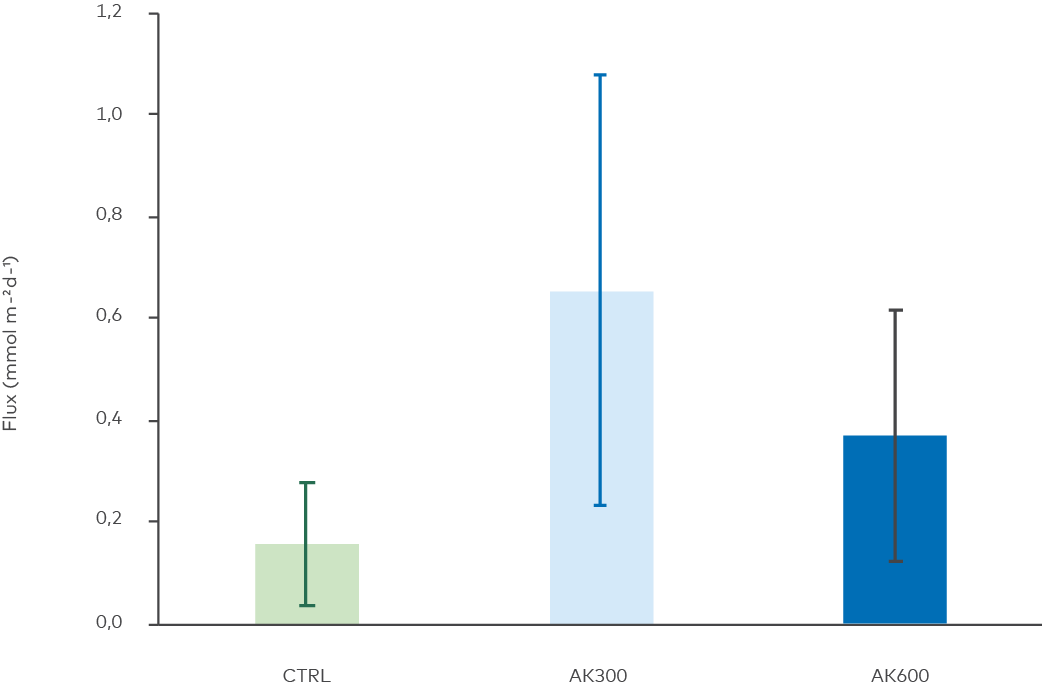
Figure 9. Effects of the treatments on sediment-to-water flux of hydrogen sulfide. No statistical difference between treatments was found (ANOVA, p = 0.09).
Since visible precipitation of iron sulfides decreased with the treatment dose, it was hypothesized that these treatments had released higher concentrations of free hydrogen sulfide. This was confirmed by analysis of core water sulfide, although the difference could not be statistically proven (ANOVA, p = 0.09). Hypothetically, this can be an effect of compromised sulfide oxidation by metals, that in turn was precipitated with hydroxide ions in the elevated pH environment. It is also possible that the energy yield of sulfate reduction was increased with the unrealistic increase in pH, as discussed above, and that sulfate reducing microbes proliferated accordingly. Our findings therefore indicate that the AK material had no impact on the amount of sulfide released from the sediment.
Respiration
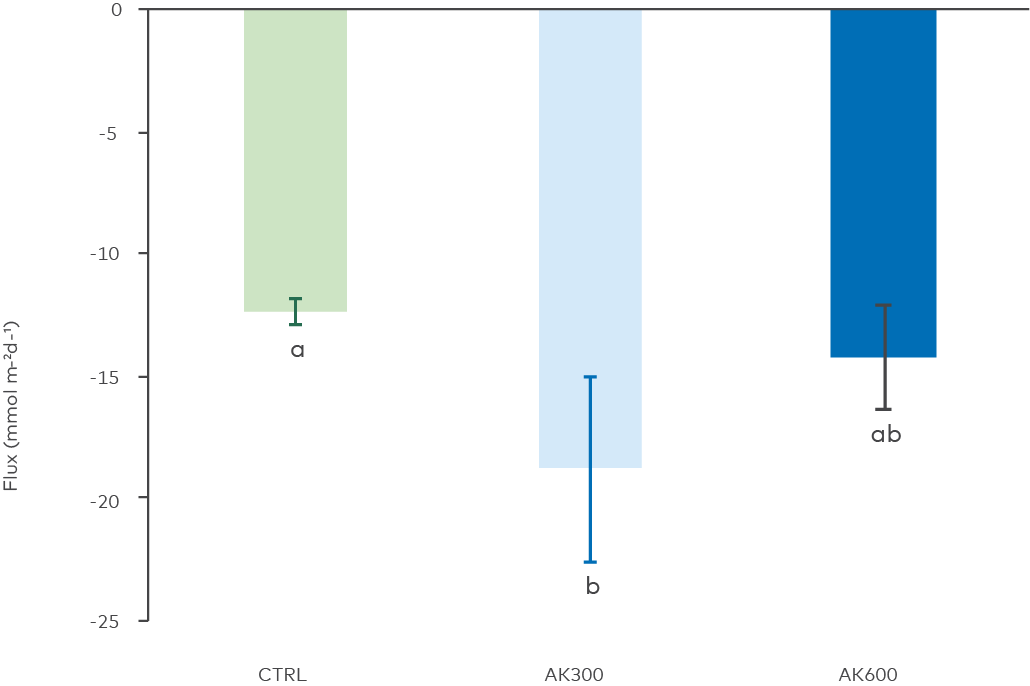
Figure 10. Total oxygen uptake by benthic microbes and fauna expressed as a negative flux. Different letters above the bars denote statistically significant differences between treatments (ANOVA, p = 0.01, Tukey’s post hoc). The AK300 treatment was found to be statistically different from CTRL.
The consumption of oxygen by microbes and benthic fauna (respiration or total oxygen uptake, TOU) was measured during the initial de-oxygenation of the cores before the anoxic incubations were done (Figure 10). The two treatments with AK showed faster oxygen consumption during this phase, and AK300 had significantly higher TOU than untreated control. This indicates that microbes and fauna in the sediment consumed more oxygen, likely due to pH-related increases in microbial metabolic rates, as discussed previously, and pH induced stress to benthic fauna.
Methane release from sediment
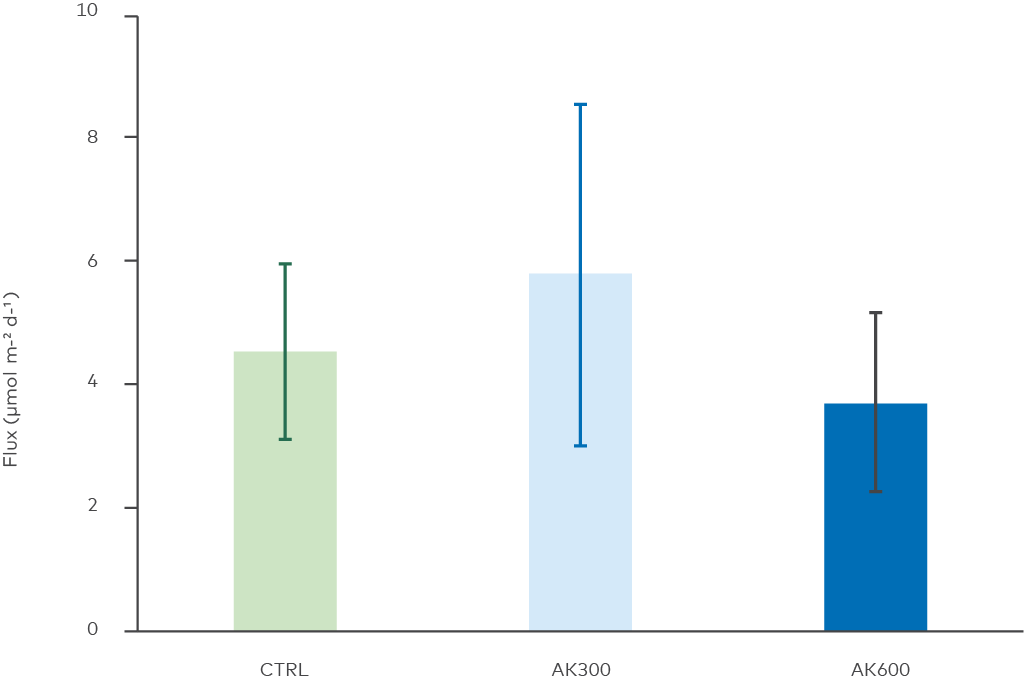
Figure 11. Methane release from the sediments. Statistical analysis showed no difference between the treatments (ANOVA, p = 0.1).
We did not detect any differences in methane release between untreated and treated sediment. Fluxes from the sediment were expected since anoxia mediates microbial methane production. However, measured fluxes were low and more in the range of fluxes from oxygenated Baltic Sea sediments (e.g., Bonaglia et al. (2014), Wikström et al. (2021). Hypothetically, methanogenesis was occurring slowly, or sufficient anaerobic oxidation of methane (AOM) degraded formed methane. Our findings indicate that the AK material did not affect methane release from the sediment.
Future perspectives
The ability of AK to retain phosphate in the sediment is a promising result that shows the potential of AK for use in remediation of eutrophic waters. A small dose of 600 g/m2 completely stopped the phosphate release from the sediment. This dose is similar to doses of aluminum chloride that have been injected in eutrophic lake sediments (Schütz, Rydin, and Huser 2017). For 16 lakes remediated with Phoslock™, an average dose of ca 300 g/ m2 was applied (Spears et al. 2013). Before such field applications, the pool of available phosphorus in the sediments is determined and the dose is calculated accordingly. Doses will therefore vary depending on the site. The AK increased porewater concentrations of phosphate which is an effect that must be studied further. However, the phosphate was entrapped in the sediment porewater in the AK treatments, possibly to small iron or other mineral hydroxides smaller than 0.45 µm in size (thus passing through the filter during phosphate measurement). Porewater phosphate can be mobilized following adverse episodes, such as resuspension caused by storms, or physico-chemical alterations following, e.g., climate change. If the increased pH caused the raised porewater phosphate, it is not likely that it would occur in a field situation where buffering capacity against pH changes is high. Furthermore, the partitioning of phosphorus, i.e., how it allocates to bioavailable forms or is trapped by the sorbent or sediment, could change in a longer experiment. A future pilot-scale study must therefore further assess these effects under a long time-period.
To summarize, this study showed that the novel activated limestone trapped phosphate in the sediment effectively, which was the purpose of the amendment, and that the material had no effects on other important sediment processes such as methane formation. There is no universal method to remediate all eutrophic waters due to their ecological and geological variability and complexity. It is, thus, important to continue to develop and test new techniques in the field.
This report was reviewed and improved by Elias Broman, Department of Ecology, Environment and Plant Sciences, Stockholm University, 2023-11-23.
This research was funded by The Nordic Council of Ministers, project number 23002.