7. Toxicity of PACs in scrubber water on pelagic organisms
A literature search was made on toxicity of PAHs and other PACs detected in scrubber water specifically on marine pelagic organisms. These organisms live in the free water masses, and therefore the ones that will encounter the highest concentrations of discharged scrubber water. The search hence included effects on fish, invertebrates that spend their entire life span in the pelagic (e.g., planktonic copepods), pelagic larval stage of invertebrates, and planktonic microalgae. Toxicity data on freshwater fish were also included to increase the data set. As already discussed, fish toxicity data are based on a better fundamental understanding of the mechanisms leading to the toxic effects, while invertebrate toxicity data are more often based on experiments where endpoints are not necessarily selected because they are known to be particularly sensitive to PAHs.
A common practice in applied ecotoxicology is to present data as the Lowest Observed Effect Concentration (LOEC), Effective Concentration 50%, 20%, or 10% (EC50/ EC20/EC10, the concentration of a toxicant having a significant toxic effect on 50%/20%/10% of the test population), or Lethal Concentration 50% or 10% (LC50/LC10, the concentration where 50%/10% of the test population dies). In this report data on LOEC have been selected as far as possible, and data on EC50/20/10 are only included when the study is of great interest, and no other data are available. Some data on mortality are included, but not when they derive from short term tests on adult individuals.
All groups of PAHs/PACs from which there are at least one compound identified in scrubber water have a heading below, even if toxicological data in many cases are scarce. The aim is not to present a resumé of the entire research field, but rather to highlight the most important findings relevant for understanding the risk of scrubber water and other oily wastewaters to the marine environment.
7.1 Toxicity of parent and alkylated PAHs
One of the most studied PAHs in terms of effects on fish embryo and fish larvae is the low molecular weight, 3-ringed compound phenanthrene, and this is also one of the PAHs that occurs at the highest concentration in scrubber water (Table 1 and 2). Embryos of the fish species marine medaka (Oryzias melastigma), exposed to low concentrations of phenanthrene for 28 days, developed a range of teratogenic effects (Table 6) (Zheng et al. 2020). Even at the lowest test concentration, 2 µg/L of phenanthrene, there was a significant increase in occurrence of yolk sac- and pericardial edema of the embryos and the time for the eggs to hatch was prolonged (Table 6). At exposure to 10 µg/L of phenanthrene the hatching success of the eggs was reduced and there were significant deformations of the heart. It should be noted that these were the nominal concentrations of the pollutant, but analyses of the test water showed that the actual phenanthrene concentrations were at least 25% lower (Zheng et al. 2020).
Fertilised eggs of zebrafish (Danio rerio) exposed to phenanthrene in low doses for 96 hours were found to develop into adults with reduced reproductive functions. Males exposed to 0.089 µg/L developed fewer mature spermatozoa and females exposed to 0.89 µg/L developed fewer mature oocytes (Table 6) (Chen et al. 2021). When adult females, exposed as embryos to 0.009 µg/L (lowest test concentration), were mating with unexposed males, their egg production was significantly reduced compared to controls. Unexposed females mating with males exposed to 0.009 µg/L produced larvae with significantly increased mortality. Females exposed to 0.089 µg phenanthrene/L had a significantly reduced fertilisation success (Chen et al. 2021).
In another study the fish species marine medaka were also exposed as embryos to low doses of phenanthrene for 96 hours (F0 generation) and allowed to mate with unexposed males and females when they reached maturity. Significant malformations of the craniofacial cartilage of the next generation of larvae (F1 generation) appeared when the male had been exposed to the lowest test concentration, 0.009 µg/L, and the female to 0.089 µg/L (Table 6) (Zhang et al. 2022).
Recent research has shown that the cardiotoxic effects of phenanthrene on early life stages of cod (Gadus morhua) greatly depends on when during the cardiac development the exposure takes place (Sorhus et al. 2023a). Hatching of cod embryos to larvae occurs 12 days post fertilisation (dpf), and it was found that when the embryos/larvae were exposed to phenanthrene from 9 dpf to 3 days post hatching (6 days in all) more severe cardiac abnormalities were observed than when the embryos were exposed from 5 to 9 dpf.
4 and 5-ringed PAHs are also toxic to developing fish, and the effect of pyrene (4-ringed), which occurs in relatively high concentrations in the scrubber water (Table 1), is found to cause malformation of the fish heart during early development. However, symptoms are not identical to those of the 3-ringed PAHs and it is believed that the toxicity, in contrast to the 3-ringed PAHs, is linked to AhR activation (Incardona et al. 2004). A study was conducted comparing the effect of phenanthrene, pyrene and benzo[a]pyrene (a 3-ring, 4-ring and a 5-ring PAH) on developing larvae of the marine fish Sebastiscus marmoratus (Table 6)(Li et al. 2011). The fish larvae were exposed to the contaminants for 8 days after fertilisation, and the most sensitive endpoints were malformation of the spine of the larvae, which occurred after exposure to 1.0, 0.1 and 0.01 µg/L of phenanthrene, pyrene and benzo[a]pyrene, respectively, and frequency of pericardial and yolk sac edema, which was detected after exposure to 1 µg/L pyrene or 0.01 µg/L benzo[a]pyrene. The lowest test concentration was 0.01 µg/L for all three compounds, and concentrations were nominal values, meaning that the actual exposure concentration could have been lower. Significant cardiotoxic effects were observed in another study on zebrafish embryos after exposure to 0.01 µg/L of pyrene (lowest test concentration) for 72 hours post fertilisation (Table 6) (Zhang et al. 2012).
Chronic exposure of zebrafish larvae to low doses of benzo[a]pyrene has been shown to have effects indicative of neurodegenerative diseases (Gao et al. 2015). After 230 days of exposure to 0.013 µg /L of benzo[a]pyrene (lowest test concentration) the swimming behaviour of the fish was significantly impaired. At 0.13 µg/L there was a significant reduction in brain weight to body weight ratio. There was no reduction in body weight between exposed and unexposed fish.
In scrubber water alkylated congeners make up around 80% of the sum of alkylated and parent PACs (Table 2). However, data on toxicity of alkylated PAHs are rare. There are studies showing that at least some alkylated PAHs are more toxic than the unsubstituted parent compound, and here phenanthrene/alkyl-phenanthrene is the most investigated homologue series (Turcotte et al. 2011, Donald et al. 2023). C1- and C2-phenanthrenes were found to be more embryotoxic (pericardial edema, yolk sac edema, hemorrhages and spinal deformities) to Japanese medaka (Oryzias latipes) than parent phenanthrene (C0) (Turcotte et al. 2011). EC50 after 17 days exposure of the fish embryos was 96 µg/L for phenanthrene and 75 µg/L for C1-phenanthrene (just one C1-congener was tested). Three different C2-phenanthrenes were included (different positions of the alkyl groups), and their EC50 were 39, 39, and 64 µg/L, respectively. EC50 for C3-phenanthrene was > 36 µg/L and for C4-phenanthrene (retene) it was 61 µg/L (Turcotte et al. 2011). In a study of developmental toxicity of phenanthrene and alkyl-phenanthrenes, embryos of Atlantic haddock (Melanogrammus aeglefinus) were exposed for 72 hours, from 2 to 5 days after fertilisation to thirteen C1-, C2-, C3-, and C4-phenanthrenes (Donald et al. 2023). No obvious difference was observed between unsubstituted and alkyl-phenanthrenes, but all compounds induced one to several morphological defects or functional impairments as measured by a set of sixteen endpoints (e.g., yolk area, body axes deformity, eye deformities, jaw deformation, heart morphology).
In addition to studies on single PAC compounds, there are also studies on effects on early life stages of fish (e.g., survival, hatching delay, hatching success, allometry, developmental abnormalities, and DNA damage) following exposure to oil, consisting of complex mixtures of PAHs/alkyl-PAHs. The results clearly indicated that the concentrations and proportions of C1-phenanthrene and C1-anthracene were the main drivers of toxicity of the mixtures (Le Bihanic et al. 2014a, Le Bihanic et al. 2014b). Strong indications that alkyl-phenanthrenes are the PAHs primarily responsible for the toxicity of crude oil to fish embryo have also been reported in other studies (Reviewed in Barron et al (2004)). Toxic effects on early life stages of fish after exposure to individual parent and alkylated PAHs are similar to what is seen after exposure to crude oil. However, the concentrations of individual PAHs causing these effects in experimental studies are much higher than their concentrations in a crude oil exposure giving rise to the same effect. The reason for this is most likely that the oil (and so also oily waters such as scrubber water) contains many more PACs with similar effects than those normally included in chemical analysis. It is also likely that mixtures of PACs are more toxic than the sum of each induvial toxic compound due to synergistic effects.
The acquired knowledge of PAH toxicity in vertebrates is not reflected in research on the effect of PAHs on invertebrates. To fish, 3-ringed PACs, specifically phenanthrene and dibenzothiophene, are found to be among the most toxic compounds in an oil spill, causing cardiac toxicity and other malformations in early life stages. Since these compounds are among the PAHs/PACs occurring at the highest concentration in scrubber water they are of great interest in the understanding of scrubber water toxicity. Studies of these compounds in invertebrates and at relevant concentrations are however limited.
Exposing adult females of copepods of the species Acartia tonsa to phenanthrene, and to the 4-ringed PAHs fluoranthene and pyrene resulted in toxic effects on the number of eggs and the time to hatching of the eggs (Bellas and Thor 2007). Pyrene was the most toxic of the compounds with the lowest EC20 being 22 µg/L for an effect on egg production, followed by fluoranthene (EC20 = 46 µg/L) and phenanthrene (EC20 = 124 µg/L) (Table 7).
In another study, the early life stages of a mussel (Mytilus galloprovincialis), a sea urchin (Paracentrotus lividus), and an ascidian (Ciona intestinalis) were exposed to naphthalene, fluorene, phenanthrene, fluoranthene, and pyrene, from the stage of newly fertilised eggs until a specific larval stage (20 – 48 hours depending on the species) (Bellas et al. 2008). The toxicity, measured as EC10 (where 10% of the exposed population is affected) was not consistent between the PAH compounds and the species. Phenanthrene was most toxic to the mussel larvae, with an EC10 of 29 µg/L, whereas fluoranthene was most toxic to the sea urchin larvae, with an EC10 of 27 µg/L (Table 7). Thereafter followed pyrene with an EC10 of 69 µg/L in sea urchin larvae, and 94 µg/L in mussels. Ascidian larvae appeared to be the least sensitive of the three species to phenanthrene, fluoranthene and pyrene, but more sensitive than the mussel and the sea urchin to naphthalene. Mussel larvae were found to be very tolerant to naphthalene with an EC10 of 4035 µg/L (Table 7).
The effect of phenanthrene was tested on larval development rate of some marine invertebrate species. The nauplii (nauplii = the first larval stage), of the copepod species Gladioferens pectinatus were exposed for six days to phenanthrene, and concentration causing a reduction in the larval development rate, measured as EC20 (the concentration where 20% of an exposed population is affected), was determined to 12.6 µg/L of phenanthrene (Table 7) (Charry et al. 2019).
A series of experimental studies have been carried out to investigate the effect of pyrene on three key species in the Arctic pelagic ecosystems, the copepods Calanus glacialis and C. hyperboreus, which are truly Artic, and C. finmarchicus which is a North Atlantic species, but all three species co-exist in the Arctic. All species build up large lipid reserves over the productive season and overwinter in resting stages, but whereas C. hyperboreus produces eggs from the lipid reserve during the winter season, C. glacialis and C. finmarchicus start producing eggs only after the onset of feeding in the spring.
Two separate long term studies were carried out where C. glacialis (Toxvaerd et al. 2018) and C. hyperboreus (Toxværd et al. 2019) were exposed to a single pulse dose of pyrene during their overwintering period, 103 days for C. glacialis and 88 days for C. hyperboreus. After exposure the animals were moved to clean water and supplied with food. Pyrene exposed individuals of both species had a significantly reduced capacity to rebuild new lipid reserves already at the lowest test concentration, 0.2 µg/L of pyrene. For C. glacialis, there was a concentration dependent decrease in survival of the animals from 0.2 to 60 µg/L of pyrene (Table 7) (Toxvaerd et al. 2018). A similar pattern was seen for the feeding rate (measured as the production of faecal pellets), and egg production, where there was a reduction already at 0.2 µg/L of pyrene. C. hyperboreus had no apparent reduction in egg production, but egg hatching success and feeding rate were both reduced at exposure to the lowest test concentration, 0.2 µg/L (Toxværd et al. 2019). The toxic effects observed in these two studies occurred at much lower concentration than in other short-term studies. This is probably because during a long-term exposure the compounds are accumulating in the lipids of the animals, and the exposure is hence not only from the external water. Data on C. glacialis was used to calculate LC50 after 103 days of pyrene exposure + 32 days recovery in clean water), and EC50 at the same time for feeding rate (measured as faecal pellet production) and egg production rate. LC50 was estimated to 5.6 µg/L, and EC50 for both feeding rate and egg production to 0.2 µg/L (Table 7) (Toxvaerd et al. 2018).
There is no data on long-term exposure of C. finmarchicus to pyrene, but in a study where copepods were exposed to pyrene for 7 – 9 days, the feeding rate of adult C. finmarchicus was significantly reduced at a concentration of 20 µg/L of pyrene, whereas this concentration had no effect either on feeding rate or on egg hatching of C. glacialis (Hjorth and Gissel Nielsen 2011). The findings on C. glacialis are hence different from those in the long-term exposure study by Toxvaerd et al. (2018) where a reduced egg production and a reduced feeding rate was found already at 0.2 µg/L.
Effects on the egg hatching and naupliar development were investigated in C. finmarchicus and C. glacialis after exposure to pyrene from the stage of fertilised egg throughout all naupliar stages (NI - NIV) (Grenvald et al. 2013). Increased naupliar mortality in both species C. glacialis occurred after exposure to 20 µg/L of pyrene (Table 7). No effect on egg hatching or hatching success was observed. This is in line with findings in Toxvaerd et al. (2018) where no effect on egg hatching was observed after exposure to pyrene for 103 days.
Studies on effects of alkylated PAHs on any invertebrate species are rare. One of the few available is a study on mortality of nauplii larvae of the brine shrimp, Artemia parthenogenetica, a common test species in standardised ecotoxicological experiments, where mortality rate was analysed after exposure to phenanthrene, C3-phenanthrene (3-methylphenanthrene), and C4-phenanthrene (retene/7-isopropyl-1-methyl phenanthrene), as well as anthracene and C2-anthracene (2-methylanthracene) (Cong et al. 2021). The animals were also exposed to benzo[a]pyrene as a “reference” substance. No clear pattern on toxicity between parent and alkylated congeners was found. Whereas C4-phenanthrene (retene) was more toxic than the parent compound phenanthrene, C3-phenanthrene was less toxic. C2-anthracene was more toxic than anthracene, which was found to have no effect on the animals. Benzo[a]pyrene was by far the most toxic of all compounds. Expressed as LC10 (death of 10% of the exposed population) after 7 days exposure, the sensitivity ranked as follows: benzo[a]pyrene (0.027 µg/L) > C4-phenanthrene (retene, 3.43 µg/L) > phenanthrene (9.76 µg/L) > C3-phenanthrene (69.6 µg/L) > C2-anthracene (87.2 µg/L) > anthracene (>402 µg/L) (Table 7).
Growth inhibition of phytoplankton is a frequently used endpoint when assessing the toxicity of compounds or mixtures of compounds, but data on toxic effects of individual PAHs on phytoplankton are rare. In a study of the effect of the PAHs fluoranthene and benzo[a]anthracene on the growth, measured as photosynthetic efficiency, of seven phytoplankton species, the sensitivity was found to vary largely between species, and it was not consistent between the two compounds (Ben Othman et al. 2012). Overall, fluoranthene was the more toxic, although the variation of the LOEC was large: between 16.1 and 161 µg/L for fluoranthene, and between 1.8 and 1825 µg/L for benzo[a]anthracene (Table 7). Isochrysis galbana was by far the most sensitive species to benzo[a]anthracene, with LOEC of 1.8 µg/L, however, this species was less sensitive to fluoranthene. Nannochloris sp. and Picochlorum sp. were the most sensitive species to fluoranthene (LOEC = 54.0 and 83.5 µg/L, respectively), and they were the second most sensitive to benzo[a]anthracene (LOEC = 57.6 µg/L).
EC50 for growth inhibition of the algae Raphidocelis subcapitata was investigated for ten alkylated PAHs, four C1-, four C2-, and two C3-congeners of naphthalene, phenanthrene, anthracene, and pyrene (Kang et al. 2016). Values could only be determined for five of the tested compounds, and one of the C1-pyrene was found to be the most toxic to the algae with an EC50 of 82 µg/L, followed by a C1-phenanthrene and a C1-anthracene (EC50 =280 and 300 µg/L, respectively) (Table 7).
PAH congener/ test species | Effect and LOEC | Reference |
Phenanthrene Fish larvae | Exposure time 96 hours Fewer mature spermatozoa in adult male: 0.089 µg/L Fewer mature oocytes in adult female: 0.89 µg/L | (Chen et al. 2021) |
Phenanthrene Fish larvae | Exposure time: 28 days Edema in yolk sac and cardium, delayed hatching: 2 µg/L Impaired hatching success: 10 µg/L | (Zheng et al. 2020) |
Phenanthrene/Fish larvae Phenanthrene/Fish larvae | Exposure time : 8 days Increased mortality: 0.1 µg/L Increased frequency of dorsal curvature:1.0 µg/L Exposure time: 72 hours Morphological changes of retina: 3.56 µg/L Exposure time 7 days: Impaired phototaxic response: 3.56 µg/L | (Li et al. 2011) (Huang et al. 2013) |
Pyrene/Fish embryo | Exposure time: 72 hours Increased frequency of heart malformation: 0.01 µg/L* | (Zhang et al. 2012) |
Pyrene/Fish larvae | Exposure time: 8 days Increased mortality: 1.0 µg/L Increased frequency of dorsal curvature: 0.1 µg/L Pericardial and yolk sac edema: 1.0 µg/L | (Li et al. 2011) |
Benzo[a]pyrene/Fish larvae | Exposure time: 8 days Increased mortality: 0.1 µg/L Increased frequency of dorsal curvature: 0.01 µg/L* Pericardial and yolk sac edema: 0.01 µg/L* | (Li et al. 2011) |
Benzo[a]pyrene/ | Exposure time: 230 days Altered swimming behaviour: 0.013 µg/L* Reduced brain/body weight ratio: 0.13 µg/L | (Gao et al. 2015) |
Exposure of embryos in the F0 generation and effects analysed in the offspring (F1 generation) of exposed individuals | ||
Phenanthrene Fish embryo/larvae | Exposure time: 72 – 96 hours Fewer eggs, increased larval mortality: 0.009 µg/L* Reduced fertilisation success: 0.089 µg/L | (Chen et al. 2021) |
Phenanthrene/Fish embryo/larvae | Exposure time: 72 hours Craniofacial malformation: 0.009 µg/L* Survival & dorsal malformation: 0.89 µg/L | (Zhang et al. 2022) |
* Effects detected at the lowest test concentration.
Table 6 PAH toxicity to fish expressed as LOEC (lowest concentration with a statistically significant effect compared to control). “Test species” indicate the life stage that was exposed to the contaminant and effects were manifested in early life stages unless otherwise indicated.
Test species/PAH congener | Toxicity | Reference |
Acartia tonsa (copepod) Phenanthrene Fluoranthene Pyrene | Egg production, EC10 48h: 124 µg/L 46 µg/L 22 µg/L | (Bellas and Thor 2007) |
Acartia tonsa (copepod) Phenanthrene Fluoranthene Pyrene | Egg hatching, EC10 48h: 187 µg/L 41 µg/L 45 µg/L | (Bellas and Thor 2007) |
Mytilus galloprovincialis (mussel) Naphthalene Phenanthrene Fluoranthene Pyrene | Larval development, EC10, 48h: 4035 µg/L 29 µg/L >253 µg/L 94 µg/L | (Bellas et al. 2008) |
Paracentrotus lividus (sea urchin) Naphthalene Fluorene Phenanthrene Fluoranthene Pyrene | Larval development, EC10, 48h: 646 µg/L 492 µg/L 460 µg/L 27 µg/L 69 µg/L | (Bellas et al. 2008) |
Ciona intestinalis (ascidian) Naphthalene Phenanthrene Fluoranthene Pyrene | Larval development, EC10, 20h: 609 µg/L >427 µg/L >253 µg/L >129 µg/L | (Bellas et al. 2008) |
Gladioferens pectinatus (copepod) Phenanthrene | Nauplii development, EC20, 6 days: 12.6 µg/L | (Charry et al. 2019) |
Calanus finmarchicus Pyrene | Naupliar mortality (exposure from egg to nauplii),LOEC: 20 µg/L | (Grenvald et al. 2013) |
Calanus finmarchicus Pyrene | Feeding rate, 7 days exposure of adults, LOEC: 20 µg/L | (Hjorth and Gissel Nielsen 2011) |
Calanus glacialis Pyrene | 103 d exposure of adults, analysed 135 days after start of exposure, LC50: 5.61 µg/L | (Toxvaerd et al. 2018) |
Calanus glacialis Pyrene, single pulse dose at beginning of exposure | 103 d exposure of adults, analysed 135 d after start of exposure, LOEC: Egg production: 0.2 µg/L Feeding rate: 0.2 µg/L Reduced rebuilding of lipid reserve: 0.2 µg/L | (Toxvaerd et al. 2018) |
Calanus hyperborealis Pyrene, single pulse dose at beginning of exposure | LOEC, 88 d exposure of adults, analysed until 17 d after start of exposure: Egg hatching success: 0.2 µg/L Feeding rate: 0.2 µg/L Reduced rebuilding of lipid reserve: 0.2 µg/L | (Toxværd et al. 2019) |
Artemia parthenogenetica Phenanthrene C3-phenanthrene C4-phenanthrene (retene) Anthracene C2-anthracene Benzo[a]pyrene | LC10,7 days 9.76 µg/L 69.6 µg/L 3.42 µg/L >402 µg/L 87.2 µg/L 0.027 µg/L | (Cong et al. 2021) |
Phytoplankton, Fluoranthene/ benzo[a]anthracene Fla/(b[a]a): Nannochloris sp. Picochlorum sp. Isochrysis galbana Dunaliella tertiolecta Chaetoceros muelleri Phaeodactylum tricornutum Alexandrium catenella | Fla/b[a]ant, Growth inhibition, LOEC 72h 16.1/57.6 µg/L 50.7/57.6 µg/L 161/1.8 µg/L 50.7/182 µg/L 161/576 µg/L 161/1852 µg/L No sign. effect/no sign. effect | (Ben Othman et al. 2012) |
Raphidocelis subcapitata,(phytoplankton) C3 (1,4,5-methyl)-naphthalene C3 (2,4,5-methyl)-naphthalene C1-phenanthrene C1-anthracene C1-pyrene | Growth inhibition, EC50 35–75 h (until steady state) 490 µg/L 470 µg/L 280 µg/L 300 µg/L 82 µg/L | (Kang et al. 2016) |
* Effects detected at the lowest test concentration
Table 7 PAH toxicity to invertebrates expressed as LOEC, EC and LC values (only average values are presented).
7.2 Toxicity of oxy-PAH
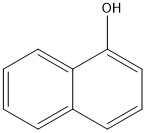
Figure 8 The oxy-PAHs detected in scrubber water from an open-loop system, from top left to right: 9,10-anthracenedione, naphthaldehyde, and phenalene-1-one, and from bottom left to right: 9-hydroxyfluorene and naphthol.
Five oxy-PAHs were detected in the non-target screening of scrubber water: 9,10-anthracenedione (synonym: anthraquinone), naphthaldehyde (synonyms; 1-formylnaphthalene; 1-naphthalene carboxaldehyde), phenalene-1-one (synonyms: phenalenone, 1H-benzonaphthene-1-one), 9-hydroxyfluorene (synonym: fluorenol), and naphthol (synonyms: naphthalen-1-ol, hydroxynaphthalene) (Fig. 8). As these were detected in the non-target screening, it was only possible to quantify naphthaldehyde. Field studies have revealed that concentrations of many oxy-PAHs are equal to, or may exceed, that of the corresponding unsubstituted PAH in collected samples of mussels and sediment (Brorström-Lunden et al. 2010, Layshock et al. 2010, De Witte et al. 2019).
Toxicity of these compounds is poorly investigated. In contrast to the parent PAHs, where genotoxicity is elicited by reactive PAH metabolites, the oxy-PAHs do not need enzymatic activation but can combine directly with DNA, RNA, and proteins once they have been taken up by an organism (Idowu et al. 2019, Qiao 2022). In vitro tests with eight commonly occurring oxy-PAHs, among them phenalene-1-one, showed that all compounds induce genotoxic effects at a similar or higher level than benzo[a]pyrene (McCarrick et al. 2019).
In another study, the non-genotoxic effects of 38 commonly occurring oxy-PAHs, including phenalene-1-one and 9-hydroxyfluorene and 9,10-anthracenedione, were tested on zebrafish embryos (Knecht et al. 2013). The embryos were exposed from 6 to 120 hours post fertilisation and a range of endpoints were analysed, including mortality, developmental progress, yolk sac edema, pericardial edema, morphology of the jaw, the eye and the body axes, fin malformation, pigment, swim bladder, and touch response. Eighteen of the tested oxy-PAHs had serious toxic effects and seventeen had moderate effects on one or several endpoints. Phenalene-1-one was among the most toxic compounds with severe effect on 8 endpoints and moderate effect on 5 endpoints at a concentration of 3,640 µg/L. 9-Hydroxyfluorene was slightly toxic at the highest test concentration, 18,200 µg/L. 9,10-Anthracenedione was among the least toxic of the tested compounds, with no effect on any endpoints, not even at the highest test concentration of 21,100 µg/L.
The toxicity of 9-hydroxyfluorene was tested on two freshwater species, the algae Scenedesmus subspicatus and the crustacean Daphnia magna and the EC50s were found to be 710 µg/L and 240 µg/L, respectively (Sepic et al. 2003).
Toxicity of 1-naphthol has been tested in a few studies on five species of freshwater fish. LC50 96 hours was found to vary between 0.33 and 4.3 mg/L (Tilak et al. 1981, Mohana Rao et al. 1984).
7.3 Toxicity of heterocyclic PAC; S-PAC
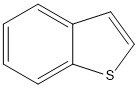
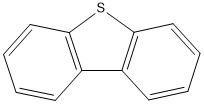
Figure 9 The two S-heterocyclic PACs benzothiophene (left,) and dibenzothiophene (right) detected in scrubber water from an open-loop system.
Two homologue series of sulphur-PACs were analysed in the scrubber water, benzothiophene and dibenzothiophene (Fig. 9). For benzothiophene, only alkylated compounds (C1-C4) were detected and for dibenzothiophene both the parent and alkylated forms (C1-C3) were found (Tables 3 and 4, Fig. 4B and 5).
Some toxic effects of dibenzothiophene resembles that of the 3-ringed PAH phenanthrene and is discussed also in the previous section, 8.1 Toxicity of unsubstituted PAH and alkylated PAH. Like the 3-ringed PAH phenanthrene, dibenzothiophene is found to be embryotoxic to fish and cause malformation of spine, yolk sac edema, pericardial edema, and eye deformation (Incardona et al. 2004, 2005, (Rhodes et al. 2005). In an experiment with embryos of the Japanese medaka (Oryzias latipes), the lowest dibenzothiophene concentration producing a significant effect (LOEC), defined as a reduced number of normal larva (defined as larvae with insignificant malformations), was 25 µg/L (Rhodes et al. 2005). The LOEC for an alkylated dibenzothiophene (C2-dibenzothiophene) in the same study was higher, 200 µg/L.
Some toxic effects of dibenzothiophene resembles that of the 3-ringed PAH phenanthrene and is discussed also in the previous section, 8.1 Toxicity of unsubstituted PAH and alkylated PAH. Like the 3-ringed PAH phenanthrene, dibenzothiophene is found to be embryotoxic to fish and cause malformation of spine, yolk sac edema, pericardial edema, and eye deformation (Incardona et al. 2004, 2005, (Rhodes et al. 2005). In an experiment with embryos of the Japanese medaka (Oryzias latipes), the lowest dibenzothiophene concentration producing a significant effect (LOEC), defined as a reduced number of normal larva (defined as larvae with insignificant malformations), was 25 µg/L (Rhodes et al. 2005). The LOEC for an alkylated dibenzothiophene (C2-dibenzothiophene) in the same study was higher, 200 µg/L.
The mechanism behind dibenzothiophene toxicity on fish embryos is independent of AhR activation and CYP1A induction, but in a study on the fish mummichog (Fundulus heteroclitus) an enhanced (synergistic) effect was found when the dibenzothiophene co-occurred with an AhR activating/CYP1A inducing compound (Wassenberg et al. 2005). Unlike in studies on zebrafish and Japanese medaka (Incardona et al. 2004, Rhodes et al. 2005), Wassenberg et al. (2005) found no effect of dibenzothiophene on the fish embryos when the exposure did not also include a CYP1A inducing substance.
Toxicity of several nitrogen, sulphur, and oxygen containing heterocyclic PACs, among them benzothiophene, were tested in a series of experiments on zebrafish embryos (Peddinghaus et al. 2012). The exposure lasted for 48 hours, starting 3 hours after fertilisation, and toxicity was measured as mortality. LC50, based on nominal concentrations of the toxicants, was 12,980 µg/L.
7.4 Toxicity of heterocyclic PAC; N-PAC
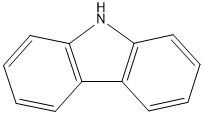
Figure 10 The N-heterocyclic compound carbazole detected in scrubber water from an open-loop system.
The N-heterocyclic compounds detected in the scrubber water were parent and alkylated (C1- C4) carbazoles (Tables 3 and 4, Fig. 5 and 10).
One of few studies on carbazole toxicity found mortality of zebra fish embryo, measured as LC50 after 48 hours exposure, to be 1070 µg/L (Peddinghaus et al. 2012). In a study of the impact of eight N-heterocyclic PACs on the growth rate of a freshwater green algae, Scenedesmus acuminatus, no effect was observed for carbazole at the applied exposure concentration and over the exposure period of 96 hours (EC10 > 400 µg/L) (van Vlaardingen et al. 1996). Four other 3-ringed N-heterocyclic PACs were considerably more toxic: acridine, EC10 = 0.32 mg/L, benzo[f]quinoline, EC10 =1.55 mg/L, phenanthridine, EC10 = 5.24 mg/L, and benzo[h]quinoline, EC10 = 6.65 mg/L.
Similar to dibenzothiophene the toxicity of carbazole to fish embryos is independent of AhR activation and CYP1A induction, but the toxicity is enhanced in the presence of another AhR activating/CYP1A inducing compound (Wassenberg et al. 2005). This synergistic effect was however found to be less pronounced than for dibenzothiophene.
7.5 Toxicity of heterocyclic PAC; O-PAC
Figure 11 The O-heterocyclic compound dibenzofuran detected in scrubber water from an open-loop system.
Two O-heterocyclic compounds, dibenzofuran (Fig. 11) and 2-methyldibenzofuran were detected in scrubber water (Tables 3 and 4, Fig. 5).
There are to our knowledge no available data on aquatic toxicity of parent or alkylated forms of dibenzofuran in their original forms. However, exposure of PAHs in general to UV radiation in sunlight leads to the formation of reactive oxygen species that cause oxidative damage in biological systems (Arfsten et al. 1996). Toxicity of photoinduced dibenzofuran has been demonstrated in the marine bacteria Vibrio fisherii, manifested as inhibition in the bacterial bioluminescence (Shemer and Linden 2007).