3. Background
3.1 Input of petroleum oil to the marine environment
Mineral oil is one of the most problematic groups of pollutants that marine organisms are confronting. It has been formed naturally over geological time spans and consists of mixtures of many thousands of organic compounds, and although many of them have the potential to be toxic, living organisms have developed physiological systems to cope with them (Goksoyr and Forlin 1992). However, the oil concentrations in the oceans today are many times higher than in pre-industrial times, and often higher than these defence systems can handle. This may lead to severe acute and chronic toxic effects.
In the 1970s and 80s there were numerous incidents of large accidental and intentional illegal oil spills at sea. Dramatic images of oil-covered birds and oil washed up on beaches were common in media. Names of some of the wrecked oil tankers, like Amoco Cadiz, Exxon Valdez and Prestige, are still familiar to many people because of the environmental disasters they caused. The attention has led to technical improvements of vessels, such as double hulls on ships, along with improved standard operating procedures. The efforts have had a positive effect on both accidental and illegal spills, and on a global basis the number has decreased form an average of 24.5 spills per year in the 1970s to 1.8 in the 2010s (Statista 2023). Instead, the most important input of mineral oil to the sea today comes from legal discharges of oil wastewaters from shipping and other maritime activities (Magnusson et al. 2018, Carpenter 2019, Lundberg 2021, HELCOM 2022). There is also ongoing leakage of stern tube oil from ships, and input of uncombusted fuel from ships and leisure boats (Johansson et al. 2020, Jalkanen et al. 2021). Until 2015 bilge water, the water that is gathered at the bottom of a ship and periodically discharged to the sea, was the most important source of oily wastewater from shipping, but since 2015 the IMO has allowed the use of scrubbers for scrubbing of the exhaust gases from ships running on heavy fuel oil (HFO), and this is now the dominating source of oil from shipping to the sea (Jalkanen et al. 2021, Ytreberg et al. 2022a). These releases of oily wastewater to the sea lead to increased base-line concentrations of oil components, primarily around ship lanes but over time also in larger sea areas surrounding the ship lanes. Oil from any future accidental or illegal oil spill will hence be added on top of these increased base-line concentrations.
3.2 Ship fuel and the IMO sulphur regulation
HFOs are the most widely used maritime fuels for vessels of all categories. HFOs are produced from heavier residuals from the crude oil refining processes and one of their main advantages over other marine fuels is that they are cheap. However, the downside is that they contain a wide spectrum of hazardous compounds and additional harmful components are formed when they are combusted. The most recognised hazardous components in the exhaust gases from combusted HFOs have been sulphur, with the acidifying effect it has when H2SO4 is formed in contact with the humidity in the air, and nitrogen oxides (NOx), with their eutrophicating properties (Stipa et al. 2007, Turner et al. 2018). The large quantities of particulate matter (PM) formed during combustion and present in exhaust gases, e.g., soot, ash, unburned organic carbon, sulphur particles, and elemental carbon (char), have also received considerable attention since many particles are of a size where they can cause damage to human lungs (Moldanova et al. 2009, Firlag et al. 2018).
To reduce these environmental threats, the IMO has passed regulations that originally involved a limitation of the sulphur content of ship fuel. Certain geographical areas regarded as particularly sensitive to acidification were designated as Sulphur Emission Control Areas (SECAs) where ship fuel, from the 1 January 2015, may contain a maximum concentration of 0.10% sulphur. The SECAs include the Baltic Sea, the North Sea and the English Channel, the United States, Canada, and the United States Caribbean Sea, and concerns all vessels over 300 gross tons (GT). A global limit for the maximum allowable sulphur content in ship fuel of 0.50% (from a previous maximum allowed level of 3.5%) was subsequently introduced on 1 January 2020 (MARPOL Annex VI, MEPC.280(70)). This regulated limitation of emission of sulphur in combustion gas is often referred to as the IMO sulphur cap. By switching to a low sulphur fuel also other hazardous components in the exhaust would be reduced. It is shown that emission factors of polycyclic aromatic hydrocarbons (PAHs, a group of PACs), oxy-PAHs and nitro-PAHs could be lowered by 97%, 77% and 73%, respectively, when using marine diesel oil (MDO) instead of HFO (Zhao et al. 2020). However, before the regulations came in place an alternative way of complying to the rules was presented. Instead of switching to low sulphur fuel, ships could install an onboard equipment, a scrubber (also called an Exhaust Gas Cleaning System, EGCS), where the exhaust gases are scrubbed with water, a process where much of the contaminants are captured in the wash water, or the scrubber water as it is also called.
There are two main varieties of scrubbers, open-loop and closed-loop. There are also hybrid scrubbers that may be operated in either an open or a closed mode. In open-loop scrubbers, the sea water is pumped onboard and sprayed on the exhaust gases. The wash water from the process, which contains most of the hazardous components in the exhaust gases, is then released back to the sea. In closed-loop scrubbers, the exhaust gases are washed with freshwater in a recirculating system with a cleaning/treatment device. Even from the closed-loop scrubbers there is a discharge of the scrubber water to the sea, although the volumes are much smaller than from the open-loop ones. In a closed-loop scrubber, part of the content in the combustion gases is retained as sludge, which is temporarily stored on-board the ship but eventually deposited on land. Nevertheless, a substantial part of the hazardous components remain in the scrubber water that is released into the sea, and since scrubber water from closed-loop scrubbers has been recirculated, the pollutant concentrations are higher than in wash water from open-loop scrubbers (Thor et al. 2021). The sulphur in the exhaust gases has a pH reducing effect, which in an open-loop scrubber is regulated with the natural buffering capacity of the sea water used for scrubbing. In closed-loop scrubbers, certain chemicals, usually caustic soda (NaOH) or limestone (CaCO3), are added to the water.
The choice to install scrubbers on-board ships instead of switching to cleaner fuel as a way to comply with the IMO sulphur cap is economically beneficial for the shipping companies. However, the economic gain has an environmental price. A consequence of the sulphur cap as it is formulated today is that a large part of the emissions of toxic compounds in the exhaust gases is transferred from the air to the sea. At the time when it was put in place, the focus of responsible authorities was exclusively on improving air quality and the terrestrial environment and no assessments of the consequences for marine ecosystems were made before this new source of pollution was introduced to the ocean (Hassellöv et al. 2020).
Scrubber water contains a considerable number of hazardous compounds. There are aliphatic and aromatic compounds from combusted and uncombusted HFO, combustion particles, residual lubrication oil from the engine, metals from the fuel oil itself, and metals released from the piping and the scrubber equipment during the scrubbing process (Kjølholt et al. 2012, Moldanova et al. 2011, (Hassellöv et al. 2020). The scrubbers remove up to 95% of the sulphur dioxide in the exhaust gas, which therefore ends up in the scrubber water instead and may contribute to local acidification of the seawater, particularly in heavily trafficked harbours and in brackish or estuarine water systems (Andersson et al. 2008, Turner et al. 2018, Yang et al. 2021). The scrubbing process is less effective at removing particulate matter (PM), measured as particles ≥2.5 µm (PM2.5) for which only about 10% of the mass was reduced (Yang et al. 2021). The particles that are not greatly affected by the scrubber process consist mainly of sulphate, followed by organic carbon and elemental carbon.
3.3 Scrubber water and the marine environment
The first, and widely cited, reports on the impact the use of scrubbers have on the marine environment claimed that pollutant concentrations in discharged scrubber water are not alarmingly high and will rapidly dilute to concentrations well below toxic levels (Kjølholt et al. 2012, IMO; MEPC 74/INF.24 2018). However, recently there has been an increasing number of both reports and scientific articles showing that scrubber water is toxic to marine biota and either recommending stricter rules for where scrubbers could be used or advocating a complete scrubber ban (Hassellöv et al. 2020, Thor et al. 2021, Picone et al. 2023). Experimental studies have shown that low concentrations of scrubber water cause severe disruption of the early development of marine invertebrates, such as planktonic copepods (small crustaceans with a central position in all marine pelagic food webs), and in larvae of blue mussels, sea urchins and polychaetes (Thor et al. 2021, Magnusson and Granberg 2022, Picone et al. 2023). In some cases, significant toxic effects occurred already at concentrations as low as 0.001% scrubber water.
Vessels equipped with open-loop scrubbers discharge wastewater from the scrubbing process (scrubber water) to the ocean as long as the engine is running. In busy ship lanes and harbours, there is therefore a continuous input of this polluted water to the environment. The number of ships equipped with scrubbers has increased over the past decade. Between 2015 and 2022, the global number of vessels equipped with scrubbers increased from 242 to 4737 (Det Norske Veritas (DNV- GL)), and in the Baltic Sea the number increased from 178 in 2018 to 462 in 2020 (HELCOM 2021). The volumes of discharged scrubber water to the ocean, and also volumes of other ship related waste streams like bilge water, grey- and black water, have been calculated using the Ship Traffic Emission Assessment Model (STEAM), where input data on activity, position and technical information of most ships in operation are obtained via the automatic identification system (AIS) (Jalkanen et al. 2021). STEAM generates data on volumes of discharged scrubber water and the geographical location of the discharges. The Baltic Sea had 462 ships equipped with scrubbers in 2020, and estimations of the volumes of scrubber water generated and discharged show that they may be as high as 0.6 billion m3/year (Ytreberg et al. 2022a).
There is a clear risk that the scrubber water pollutants will be harmful to the Baltic ecosystems. Pelagic organisms are the primary target since they are found where the scrubber water is discharged, but benthic communities and sensitive coastal habitats near ship lanes will also be affected. Many stretches of shipping lanes in the Baltic Sea run close to coast lines or shallow sea areas, e.g., in the Gulf of Finland, south of Gotland, the Oresund Sound and the Belt areas. In all these areas there are zones which are important breeding and nursery grounds for fish, stopover sites for huge numbers of migrating birds feeding on invertebrates in shallow water, areas of great importance for over wintering sea birds, etc. (Larsson 2012, Sundblad and Bergstrom 2014, Kraufvelin et al. 2018). To these ecosystems the dilution of scrubber water in the sea might not necessarily be enough to keep concentrations of potentially hazardous components at safe levels. As an example, embryos of haddock developed heart malformations after exposure to diluted crude oil where the concentration of total PAHs amounted to 0.1 µg/L, which corresponds to a dilution of around 0.001% scrubber water (Sorhus et al. 2023b).
3.4 Polycyclic aromatic compounds (PACs) – one of the most toxic fractions of scrubber water
Classes of PAC
PACs are compounds with at least two benzene rings or benzene-like rings fused together. They include the better known polycyclic aromatic hydrocarbons (PAHs) but encompass also compounds with nitrogen, sulphur, or oxygen in the ring structure i.e., the heterocyclic PACs. In addition, PACs can be alkylated or contain different functional groups, allowing the PACs to include thousands of compounds. PACs without alkylation or functional groups are often referred to as parent compounds or unsubstituted compounds, while PACs with alkylation or functional groups are referred to as substituted compounds (Boehm 1964, Stout et al. 2015).
Unsubstituted PAHs receive most attention in risk assessments and in ecotoxicological research, but when alkylated PAHs are included in chemical analysis of environmental samples affected by oil spills, they are often found to occur at higher concentrations than the unsubstituted PAHs (Golzadeh et al. 2021, Grung et al. 2021). Alkylated PAHs can have one to several alkyl groups attached to the ring structure. The parent PAH and the corresponding alkylated PAHs form a “homologue series”, where each compound group has a prefix indicating the number of carbon atoms in the attached alkyl groups. A C0-PAH is hence the parent PAH, C1-PAH is a PAH with one methyl group, a C2-PAH has two methyl groups or one ethyl group, a C3-PAH has three methyl groups, one methyl group and one ethyl group, or a propyl group, and C4-PAH may have various combinations of methyl- ethyl-, and propyl groups or a butyl group. The position of the alkylation(s) on the ring structure and the various combinations of alkyl groups generate numerous isomers of alkylated PAHs. Besides the alkylated PAHs there are PAHs with an oxygen or nitrogen containing functional group attached to the parent compound. Alkyl- and oxygen containing PAHs are abundant in petroleum and oil while nitrogen- and oxygen containing PAHs are formed during incomplete combustion (Zhao et al. 2020, Goto et al. 2021).
In the 1970s, a list of 16 PAHs, consisting of compounds with 2 – 6 aromatic rings, were selected by the United States Environmental Protection Agency (US EPA) to be used when assessing risks to human health from drinking water (Andersson and Achten 2015a). The 16 US EPA PAHs were selected for three reasons: there were existing analytical standards available, they were known to occur in the environment, and they were known to be toxic. The intention was not that they should be used as a standard reference set for general risk assessment (Keith 2015). Yet this is the case for example in the EU Water Framework Directive where all PAHs that are regulated belong to the 16 US EPA PAHs (Directive 2013/39/EU). This practice has resulted in a situation where numerous PACs with potential toxic effects, such as alkyl-, oxygen-, and nitrogen containing PAHs, are not regulated at all.
Fate of PACs in the marine environment
PACs are hydrophobic, semi-volatile compounds with a high affinity for organic surfaces, including organic particles, living organisms, and biofilms. In general, hydrophobicity increases with increased number of aromatic rings and increased molecular weight. Alkylated PAHs are more hydrophobic than their corresponding parent PAHs, and the hydrophobicity increases with the degree of alkylation. Since particle affinity is directly correlated to hydrophobicity, most PACs compounds in the environment are extensively adsorbed to the surface of particles, primarily organic ones, and will be transported along with them. Factors like wind conditions, waves, sea currents and stratification of the sea water, will hence have a large influence on the spread of PACs from a point source.
The uptake of PACs and other organic pollutants by living organisms occurs simply by passive diffusion through the lipid-rich cell membranes. The uptake can be from the surrounding water over the gills or body surface, or over the gut epithelium when the animals consume PAC contaminated food. Through evolution, organisms have adapted a metabolic capacity to handle PACs and prevent them from accumulating in the body. Although this capacity is present in all living organisms, the metabolic efficiency differs considerably between groups of organisms, with invertebrates having a significantly lower capacity than vertebrates (Livingstone 1998). The PAC metabolites formed through the metabolic turnover are more water-soluble than the parent compounds and can therefore be excreted with urine (or similar, depending on the species). Many of the PAC metabolites are harmless, but there are also reactive metabolites formed in the process, and these are responsible for an important part of PAC toxicity. This means that a higher metabolic rate and exposure to higher PAC concentrations will generate more reactive metabolites and subsequently increase the risk of adverse effects. There are some data on trophic transfer (from prey to predator) of pyrene metabolites between invertebrate species (Carrasco Navarro et al. 2013). It is however not known how important this transfer is.
A consequence of the efficient metabolic turnover rate, especially by species at higher trophic levels is that PACs do not biomagnify in aquatic food webs. This is in contrast to halogenated organic contaminants like polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs) and dichlorodiphenyltrichloroethane (DDT) for which biomagnification is the result of slow metabolic turnover rates allowing concentrations to increase by each trophic level (Borgå et al. 2001, Takeuchi et al. 2009). The fairly efficient metabolism of PACs means that PAC concentrations in organisms do not correspond well with the exposure situation. A low PAC tissue concentration in an organism is not necessarily indicative of low PAC concentrations in the environment but instead the result of an efficient turn-over rate. The lack of biomagnification was demonstrated very clearly in a Baltic Sea food chain, where 15 parent PAHs and 4 alkylated PAHs were analysed in seston (= plankton and organic particles), in blue mussels (Mytilus edulis) which feed exclusively on seston, and in gallbladder of young eiders (Somateria mollissima), which feed almost exclusively on blue mussels (Broman et al. 1990). All PAH concentrations were found to decrease when moving up the food chain, from seston to blue mussel to eider (Fig. 1). Similar results were found in a study where PAHs and PCBs were analysed in field-collected species of mussels, crustaceans, and fish from the innermost part of Tokyo Bay in Japan (Takeuchi et al. 2009). In this study the trophic position in the food web of each species was determined using a δ 15N, a method well established to use for this application (Hobson and Welch 1992) and it was found that whereas the PCB concentration increased significantly with increased trophic level, the PAH concentration decreased (Fig. 2). Most studies on metabolism of PACs are carried out on PAHs, and there are limited data on if and how the metabolic activity differs between different groups of PACs.
Figure 1 Concentration of unsubstituted and alkylated PACs in a Baltic marine food chain consisting of seston (=plankton/organic particles; n=10 ), blue mussel (n= 6 ), and juvenile eider (n= 3 ). A) Concentrations of 15 parent PAHs; B) Concentrations of 4 alkylated PAHs. Data obtained from Broman et al. 1990.
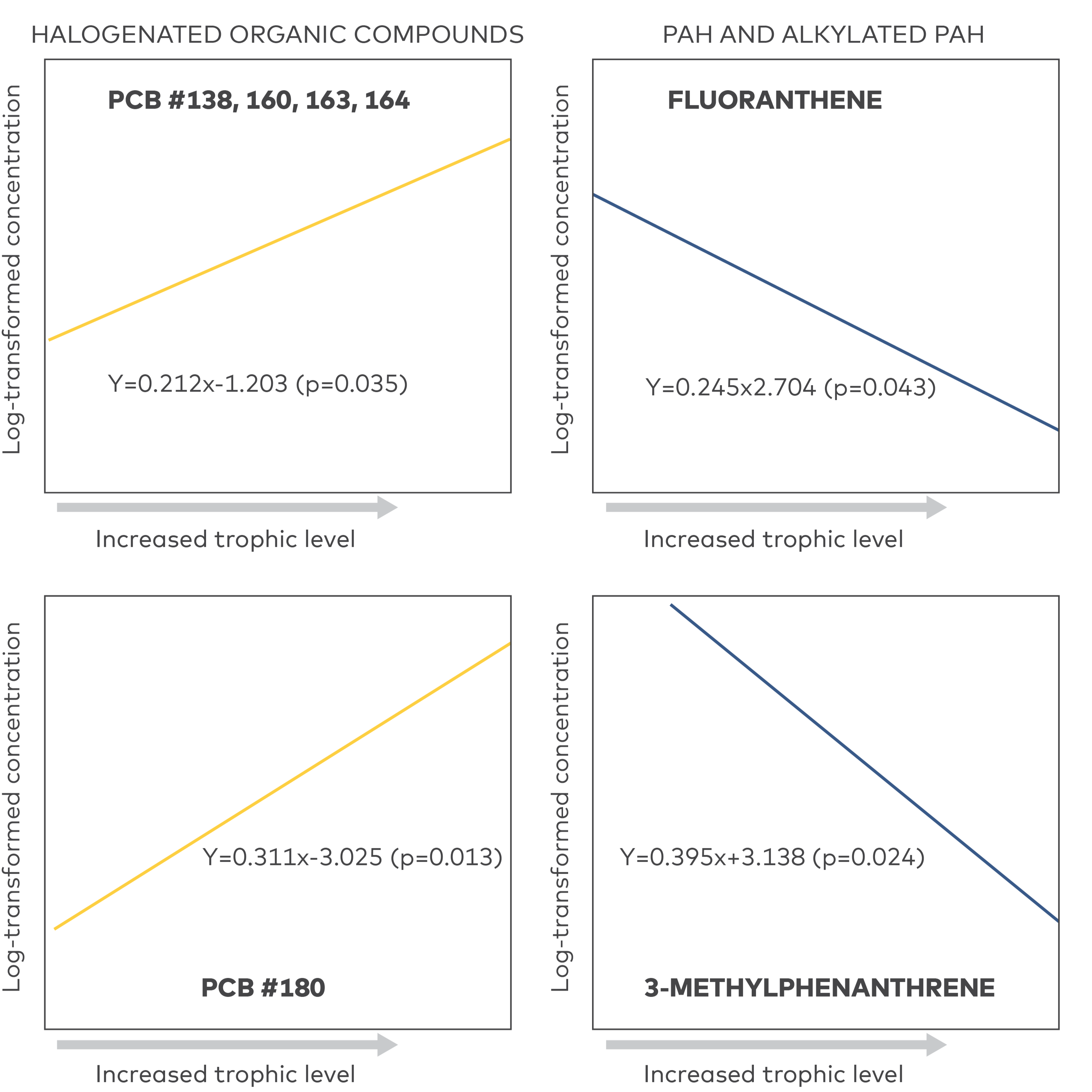
Figure 2 Concentrations of two PCBs, one PAH (phenanthrene) and one alkyl-PAH (3-methyphenanthrene) in organisms at different trophic levels (mussels, crustaceans, fish) collected from the innermost part of Tokyo Bay, Japan. Figures are redrawn from Takeuchi et al. 2009.