6. PAC fate and general toxicity
PACs are considered to be the most toxic group of compounds in scrubber water, and there is an extensive amount of research going on covering effects and underlying mechanisms at genetic, molecular, cellular, and tissue levels, as well as effects that can be observed in studies on whole-organism or even at population and community levels. The PACs consist of hundreds of compounds, but only a few of them, mainly some of the 16 US EPA PAHs, receive attention in research or in legislation (e.g., development of standardised tests or development of threshold values for acceptable concentrations in the environment or in food for human consumption). Data on toxicity of alkylated PAHs, oxy- and nitro-PAHs, and on heterocyclic PACs are scarce. In a review on the effect of PAHs on reproduction in all animals, 75 relevant articles were found, and the total number of PAHs in the described experiments included five PAHs (i.e., benzo[a]pyrene, pyrene, phenanthrene, fluoranthene, and naphthalene) and one group of methylated PAHs (i.e., C2-naphthalene) (Bolden et al. 2017). Despite the limited knowledge on most PACs, it is clear that there is a significant difference in toxicity between individual compounds. There is also a difference in sensitivity between species and between different life stages of a species, with early development generally being the most vulnerable. Data show that early life stages of fish are very sensitive to PACs, and effects are often detected at lower concentrations than effects in marine invertebrates (Tables 6 and 7). Whether this really means that fish are more sensitive or whether it is a result of more research being done on vertebrates is not clear. Some toxic mechanisms occur only after the PAH molecule has been metabolised, and since vertebrates have a more efficient PAH metabolism this could partly explain the higher sensitivity.
The extensive research on PAH and other PACs is in sharp contrast to the limited data commonly referred to when assessing the toxic effects of scrubber water on marine ecosystems. These data generally derive from standardised tests/assays of a few PAHs with the most frequent endpoint being mortality in a population after 24 – 96 hours exposure to the toxicant (European Commission 2003). The same kind of tests also form the basis for developing pollutant threshold values, e.g., the EU environmental Quality Standards (EQS) (Directive 2013/39/EU). Threshold values only exist for a few PAH congeners and are rarely re-evaluated and updated according to new scientific findings. EQS for the EU priority pollutants have not been updated since 2013.
Sea water around the Nordic countries generally have pollutant levels well below all these EQSs, including those for the PAHs. Still, it is very clear that the marine ecosystems are not doing well, and the situation deteriorates rapidly with declining populations of virtually all marine species in most sea areas. No area of the world’s oceans is completely unaffected by man, and it is estimated that up to 40% is heavily affected (Halpern et al. 2008, Crain et al. 2009). The problem is multifaceted, and the role of PACs and other oil derived pollutants is not at all clear. It is however well documented that this group of compounds has a wide range of biological effects that may appear already after exposure to very low concentrations. In marine ecosystems these effects are best documented on early life stages of fish and include morphological deformations, cardiotoxicity, mutations and cancer, oxidative stress, endocrine disruption, impaired reproduction, mortality rates of embryos, reduced growth, circulatory failure, and fin erosion (Billiard et al. 2008, Hodson 2017, Wallace et al. 2020). It can therefore not be excluded that scrubber water, this new source of PACs to the ocean, could have an impact on the already hard-pressed coastal marine ecosystems.
Although there is an extensive scientific literature on PAH toxicity (data on other groups of PACs are scarce), knowledge is limited to a comparatively small number of individual compounds, and the toxicity is found to vary considerably between different groups of organisms. Research on the physiological mechanisms behind PAH toxicity has primarily been done on vertebrates, including fish. Studies on PAH in fish have been carried out for over half a century and developed into a large and well-established field of research, while data on toxicity on invertebrates and algae are more scattered. Therefore, when conducting toxicity studies to investigate the effects of scrubber water, there are a number of endpoints in fish known to be sensitive to PAHs present in scrubber water, while it is less clear what would be the most sensitive endpoints in invertebrates and algae. Available data on the effect of PAHs on invertebrates include studies on fertilisation, egg hatching, and early life stages, and although it has been shown that some life stages are more sensitive than others, the observations are rarely used for further studies on the underlying mechanisms. This limitation in knowledge on PAC toxicity on invertebrate poses a risk that toxicity studies on invertebrates are not carried out at the most sensitive life stages and that the risk scrubber water, and other oily wastewaters, pose to the marine environment is underestimated.
PAHs are metabolized more efficiently than halogenated organic pollutants (Takeuchi et al. 2009). However, in the metabolic process also reactive and potentially toxic metabolites are formed. This means that metabolism of PAHs is at the same time a detoxification process, by eliminating the PAHs, and a generator of toxic PAH metabolites. Formation of reactive metabolites is not the only toxic pathway, but studies in fish and other vertebrates have shown that PAH toxicity is caused by multiple mechanisms, varying between compounds, where important factors determining the toxic mode of action are e.g., the number of rings, number of alkyl carbons, and the steric composition of the compound (Hodson 2017, Incardona 2017). These multiple modes of action behind PACH toxicity make it difficult to theoretically predict the effect of PAHs mixtures.
Metabolism of PAHs is a two-step process. There is a phase I where the parent compound is metabolised to intermediate metabolites, and a phase II where these metabolites bind to conjugating enzymes and form water soluble conjugates with low toxicity that can be excreted (Varanasi 1989, Franco and Lavado 2019). Phase I is initiated when PAH molecules that have passed through the cell membrane, bind to, and thereby activate, the aryl hydrocarbon receptors (AhR) which is present in the cytosol (Fig. 7). The activated PAC-AhR complex controls the expression of a battery of genes in the DNA of the cells that encode for enzymes that convert PAH into water-soluble metabolites (phase II) (Nebert et al. 2004). One important family of PAH metabolising enzymes is the cytochrome P450 1A (CYP1A), and this and other enzyme systems are induced by many (but not all) PAHs. The phase I and phase II metabolic pathways prevent PAHs from accumulating, and the induction of CYP1A enzymes is a way for animals to adapt to situations of exposure to high PAH concentrations. However, phase I also generates the formation of reactive metabolites that may bind to cellular macromolecules and have a range of toxic effects, including mutations, cancer, immunotoxicity, teratogenic, and anti-estrogenic/anti-androgenic effects (Nebert et al. 2004, Bolden et al. 2017). So, a higher CYP1A production as a response to increased PAH/PAC exposure leads to an increased production of both harmless metabolites, and reactive metabolites that may cause different kinds of damage to the organism. From an ecotoxicological perspective it is therefore important to keep in mind that an increased PAH exposure will not necessarily lead to an increased bioaccumulation of PAHs (Fig. 1 and 2), but there will be an increased production of the toxic PAC metabolites.
The induction of CYP1A can be used in field monitoring of PAHs. Since these compounds are not bioaccumulating to any great extent, exposure to them can instead be estimated by measuring the CYP1A activity.
The AhR/CYP1A related mechanism of PAH toxicity is similar to that of dioxins and is mainly important for high molecular weight PAHs. Low molecular weight PAHs do not bind to the AhR but exert their toxicity through other pathways. Several of the low molecular weight (3-ringed) compounds are known to cause disturbance of cardiovascular function and craniofacial and body axis defects in fish, and the mechanism behind this is suspected to be an effect on the ion regulation in cells (Incardona et al. 2005, Incardona 2017).
Since at least part of the toxic effects of PAHs are linked to their metabolic conversion, species with a high metabolic capacity could be suspected to be more sensitive to these compounds than species with lower capacity. Invertebrates have a considerably lower metabolic turnover of PAHs than fish and other vertebrates (Livingstone 1998), and scientific data indicate that they also are less sensitive (see Tables 6 and 7 for references). However, it cannot be ruled out that this merely reflects the fact that there is much more research on effects of PAHs on fish than on invertebrates.
In addition to the specific toxic mechanisms mentioned above (through AhR activation and disturbance of ion channels), PAHs also have been claimed to have a non-specific mode of action, often referred to as “narcosis” (Di Toro et al. 2000). Narcosis is suggested to be caused by the mere presence of the hydrophobic PAC molecules dissolved in the cell membranes which would disturb normal cell processes and result in an anaesthetic or narcotic-like effect. With increasing molecular weight, PAH molecules become more hydrophobic, and this would lead to an increased narcotic effect. Lately the evidences for this form of narcotic effect have been questioned (Incardona 2017), although it cannot be ruled out that it exists.
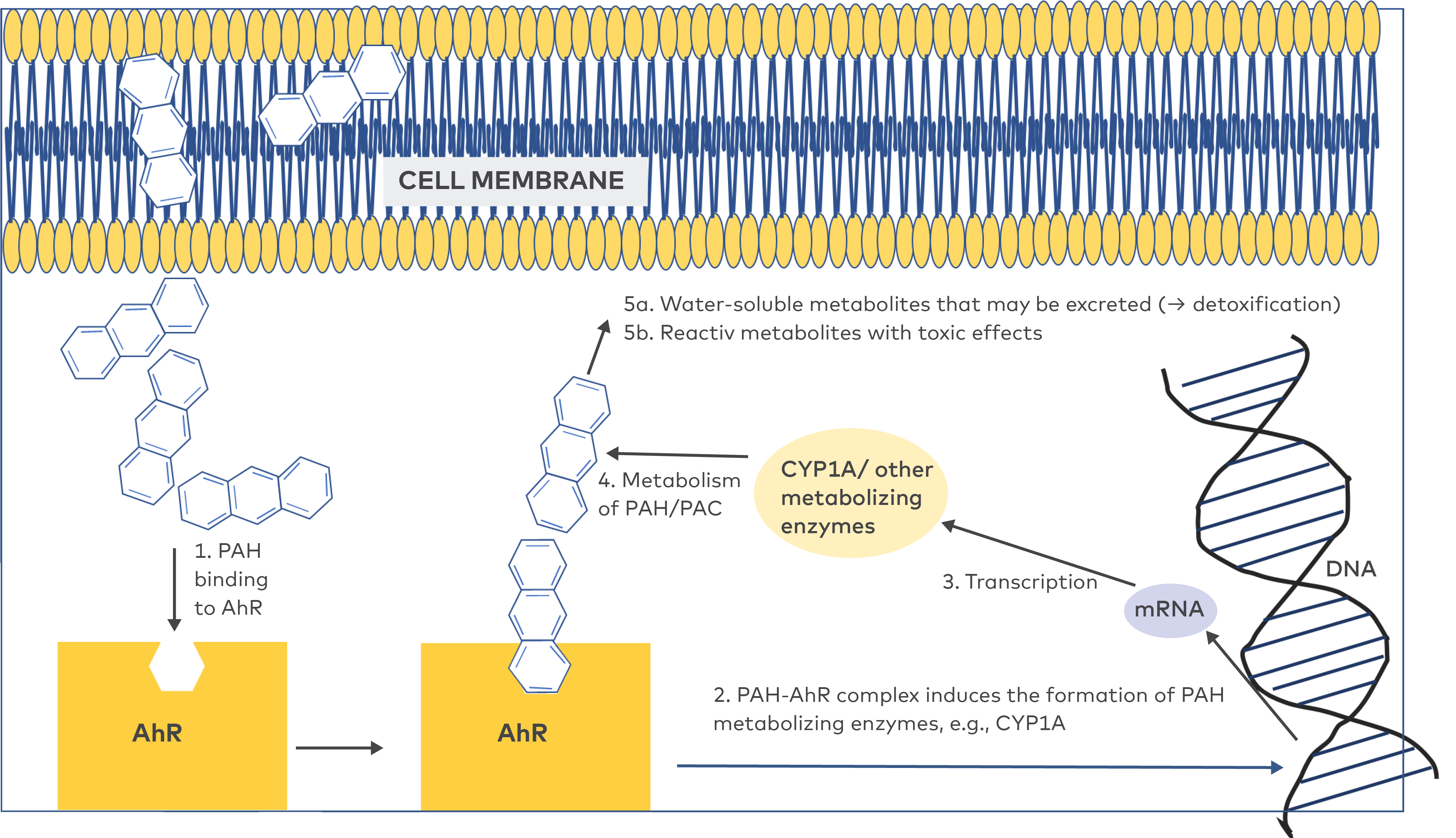
Figure 7 The metabolic turnover eliminates PAHs and limit the accumulation in the organism, but at the same time reactive metabolites with toxic effects are generated.