4. Potential process for determining and listing PUA plastic products
4.1 A simple decision hierarchy for determination
Figure 6 provides a simple decision hierarchy for determination of problematic, unnecessary and avoidable plastic products. Three decision points are provided to help prioritise actions towards a safe and sustainable plastics economy, based on the criteria presented in Section 3.
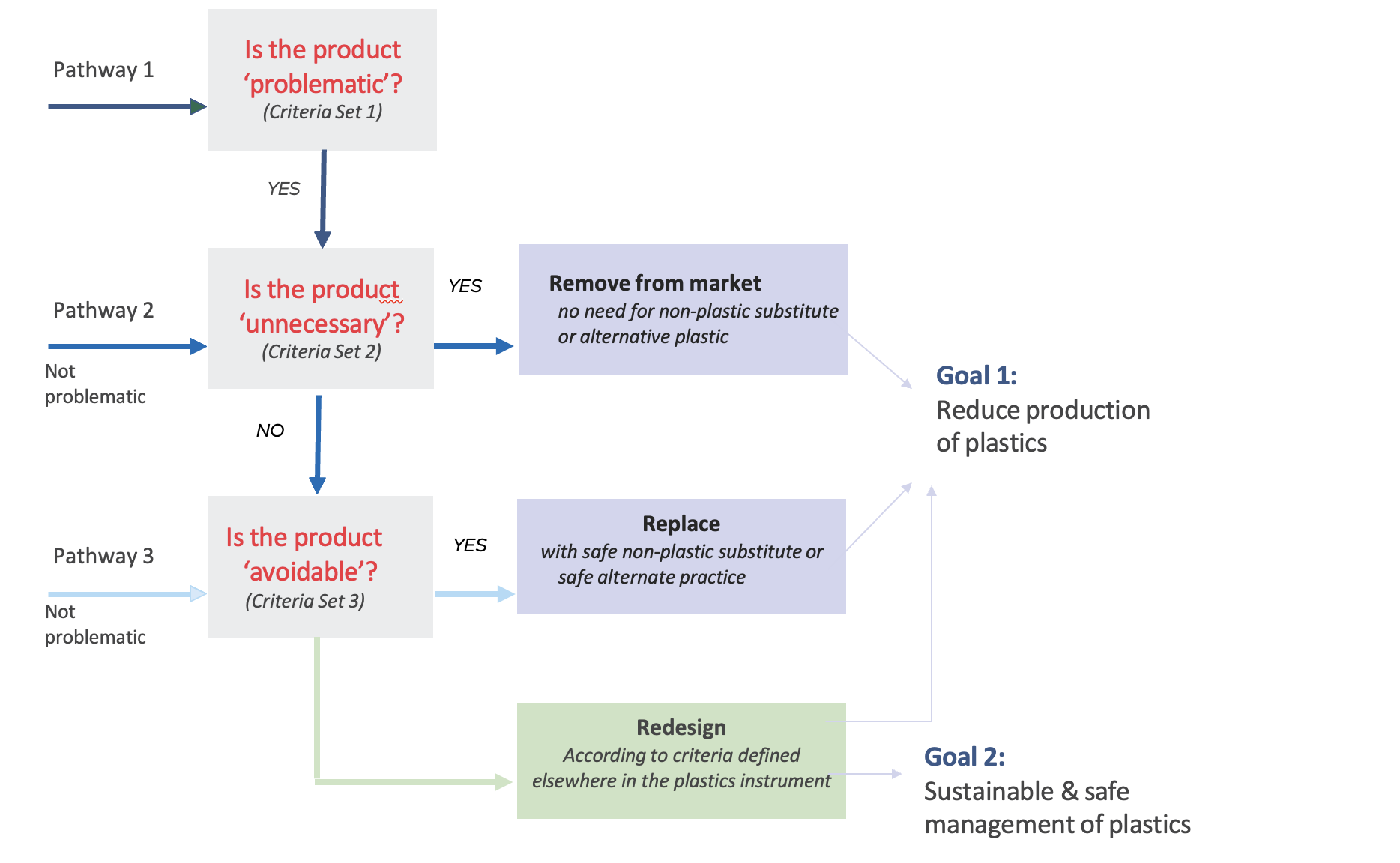
Figure 3. A simple decision tree to determine actions to address problematic, unnecessary and avoidable plastic products
Products that are not considered “problematic” could follow pathways 2 or 3. All actions should contribute to the two main goals of:
- Goal 1 – Reduce production of plastics through the removal of unnecessary products from the market, replacement with safe non-plastic substitutes and alternate business practices.
- Goal 2 – Sustainable and safe management of plastics that remain on the market through redesign according to sustainability criteria, including safe alternatives, and circular business practices.
Figure 8 illustrates the desired outcomes of the criteria for problematic, unnecessary, and avoidable plastic products. If the product fits only into the problematic classification, these products should be prioritised for redesign. The figure also illustrates that problematic plastics can overlap with unnecessary products, potentially changing their management action to removal. Similarly, where problematic products overlap with avoidable products, their management action could change to replacement, possibly also requiring redesign.
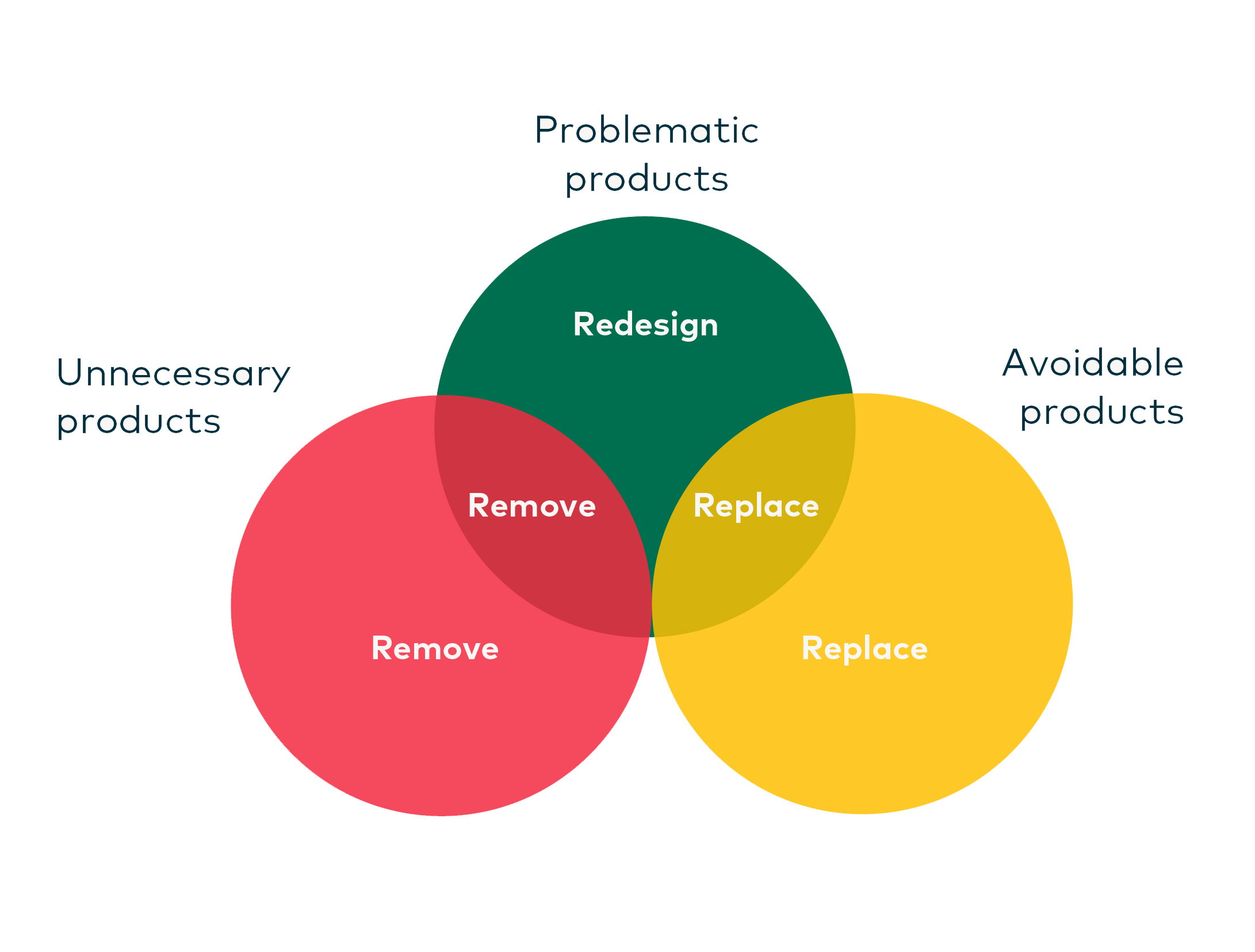
Figure 4. Desired outcomes of criteria for problematic, unnecessary and avoidable plastic products
4.2 Product exceptions and exemption processes
Product exceptions, such as acceptable purposes and essential uses, are provided for in existing MEAs and allow for special cases in which restrictions would not apply. See Box 2 for examples of acceptable purposes and essential use processes under the Stockholm Convention and the Montreal Protocol.
Under special conditions, parties may be granted time-bound exemptions for restricted products. This would allow Parties to seek alternatives to the restricted product, to extend the phase out period for uses of regulated substances or meet other requirements as per the MEA.
Product exceptions and Party exemptions under the plastics instrument could be granted based on a life cycle and socio-economic analysis and could consider the following criteria:
These criteria are suggested by the authors based on criteria in existing MEAs for determining essential uses, exceptions and exemptions, as well as comments from the Advisory Group. See Box 2 for examples from existing MEAs.
- Socio-economic benefits must outweigh risks to human health and the environment,
- No suitable alternative substances or technologies are available, and
- Additional control measures that can be implemented to minimize the impacts.
Box 2: Examples of exemption processes under MEAs.
The Stockholm Convention provides a mechanism for determining time-limited “specific exemptions” and time-unlimited “acceptable purposes” for particular use(s) and production of chemicals otherwise restricted by the Convention’s control measures. The granting of such exemptions is informed by recommendations from the Persistent Organic Pollutants Review Committee (POPRC) based on a risk management evaluation, ensuring a scientifically credible approach to exemptions (see section 5). Should alternatives not exist or not be readily available for regulated substances, Parties can still continue their specific use by registering for a specific exemption or acceptable purpose as per Annexes A and B to the Convention. Article 4 establishes a registry for this purpose, listing the specific exemptions under Annexes A and B, the Parties that have specific exemptions and the expiry dates for each registered exemption. All registrations of specific exemptions expire five years after the Convention entered into force. A review process is in place for each exemption registered and Parties wishing to extend the exemption may submit a report to justify this. The extension may be for up to five years and a Party may withdraw a registry entry at any point, with no new registrations allowed should there no longer be any registrations for a specific exemption. Parties that have registered a specific exemption or acceptable purpose as per Annex A or B must prevent or minimise human exposure and releases to the environment from production or use (Art. 3.6).
Parties to the Montreal Protocol have adopted various Decisions on essential uses.
- A use of a controlled substance should qualify as “essential” only if:
- it is necessary for the health, safety or is critical for the functioning of society (encompassing cultural and intellectual aspects); and
- there are no available technically and economically feasible alternatives or substitutes that are acceptable from the standpoint of environment and health.
- Production and consumption, if any, of a controlled substance for essential uses should be permitted only if:
- all economically feasible steps have been taken to minimize the essential use and any associated emission of the controlled substance; and
- the controlled substance is not available in sufficient quantity and quality from existing stocks of banked or recycled controlled substances, also bearing in mind the developing countries’ need for controlled substances.
- Production, if any, for essential use, will be in addition to production to supply the basic domestic needs of the Parties operating under paragraph 1 of Article 5 of the Protocol prior to the phase-out of the controlled substances in those countries.
In addition to defining the criteria for determining “essential use” in Decision IV/25, the Technology and Economic Assessment Panel and its Technical and Economic Options Committee is requested to make recommendations on nominations by Parties based on the above criteria and considering the environmental acceptability, health effects, economic feasibility, availability, and regulatory status of alternatives and substitutes. Recommendations must address:
- “the essential use (substance, quantity, quality, expected duration of essential use, duration of production or import necessary to meet such essential use),
- economically feasible use and emission controls for the proposed essential use,
- sources of already produced controlled substances for the proposed essential use (quantity, quality, timing), and
- steps necessary to ensure that alternatives and substitutes are available as soon as possible for the proposed essential use.”
4.3 Application of criteria at the global and national levels
To strengthen the global governance of plastics, criteria developed under the plastics instrument could act on two levels by stimulating both mandatory and voluntary measures (Figure 5):
- Mandatory measures for listed products: At the international level, criteria provide the basis for identifying, nominating and listing products, potentially within annexes to the plastics instrument. Once a product is listed, related control measures mandate or promote/encourage national action by Parties to the instrument. As designed in other MEAs, the mandatory control measures could include three elements:
- Bans and restrictions to be taken by Parties for products listed at the global level.
- Trade measures between Parties and with non-Parties of listed products.
- Time-restricted exemptions for products as deemed necessary on a case-by-case basis.
- Voluntary measures for non-listed products: At the national level, Parties may use global criteria to voluntarily identify additional products not listed or regulated by the plastics instrument and take action beyond the obligations established under the instrument, thereby strengthening national action compared to the requirements of the instrument.
Additionally, voluntary national listings could be collated into a global database for candidate products administered by the Secretariat of the plastics instrument. This could provide 1) an opportunity to identify candidate products that can in future be considered for global listing under the plastics instrument or 2) may inform strengthened voluntary action at the national level.
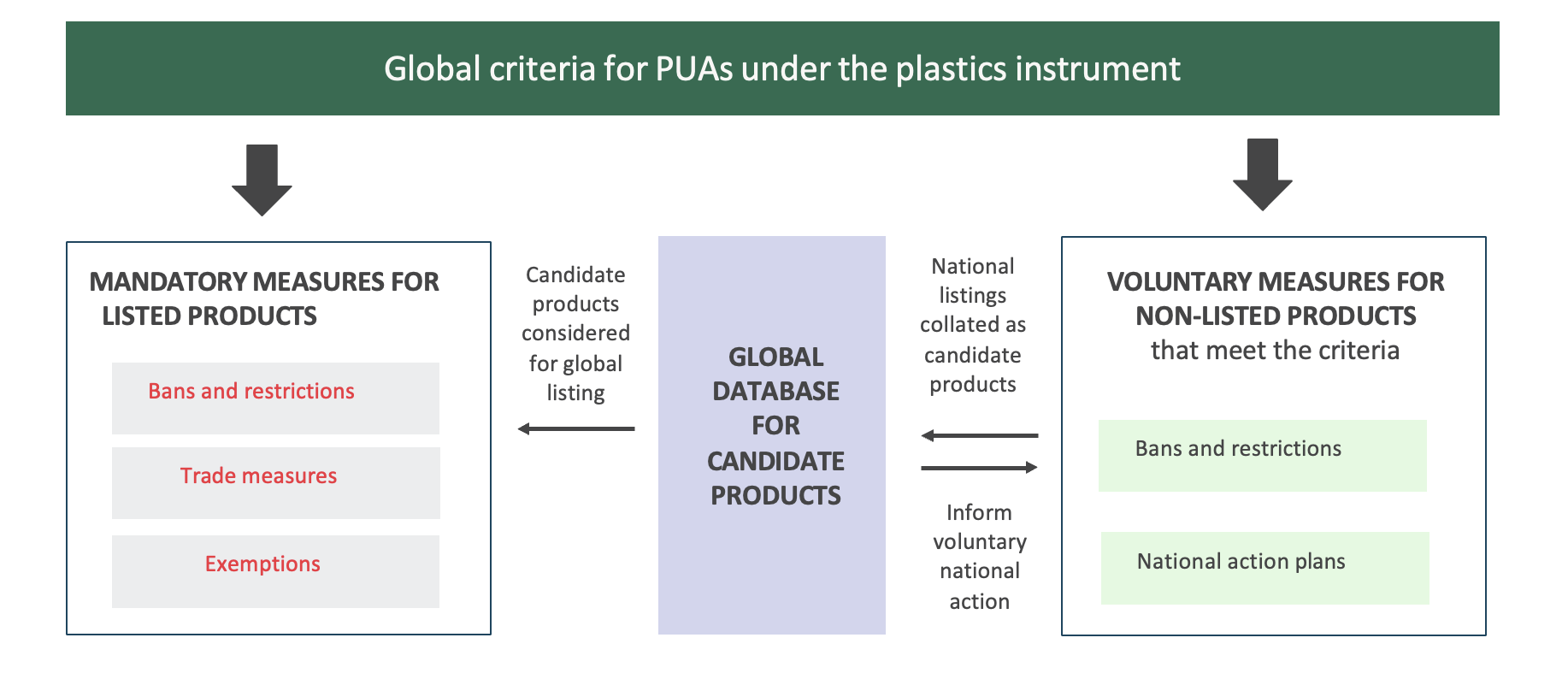
Figure 5. The role of global criteria for PUA products under the plastics instrument in stimulating both mandatory and voluntary measure.