

- Full page image w/ text
- Authors
- Table of contents
- Executive summary
- List of Acronyms
- 1. Introduction
- 2. Background
- 2.1. Biodiversity and Climate Change in the Nordic Region
- 2.1.1. Peatlands
- 2.1.2. Forests
- 2.2. Approach and methodological considerations
- 2.2.1. Biodiversity
- 2.2.2 Climate
- 2.3. International policy agreements
- 2.3.1. Sustainable Development Goals (SDGs)
- 2.3.2. The Conventions on Biodiversity and Climate Change
- 2.3.3. Other global agreements and science-policy platforms
- 2.3.4. The European Union
- 2.3.1. Sustainable Development Goals (SDGs)
- 3. Synergy between biodiversity and climate regulation
- 3.1. Peatlands
- 3.2. Forests
- 4. Nordic Policy options
- 5. Introduction to case studies
- 6. Case studies
- 6.1. Restoration of one of the largest raised bogs in lowland northwest Europe, Denmark
- 6.2. Restoration of natural landscape hydrology - mire restoration, Sweden
- 6.3. Palsa mires – a threatened sub-arctic habitat, Norway
- 6.4. Future land use options in peatlands with abandoned forestry, Finland
- 6.5. Natural regeneration of temperate deciduous forests, Norway
- 6.6. Restoration of an almost extinct forest ecosystem, Iceland
- 6.7. Doubling the area of forest set aside biodiversity for conservation, Denmark
- 6.8. Development of biodiversity and carbon storage in ancient boreal forests, Sweden
- 7. Acknowledgement
- References
- About this publication
MENU
Synergy in conservation of biodiversity and climate change mitigation in Nordic peatlands and forests
– Eight case studies
Synergy in conservation of biodiversity and climate change mitigation in Nordic peatlands and forests. Eight case studies.
Lars Dinesen1
Anders Højgård Petersen2
Carsten Rahbek1,2
IPBES in Denmark
Center for Macroecology, Evolution and Climate
Nordic Council of Ministers
Contents
Executive summary
We are facing two global environmental crises, the loss of biodiversity and climate change. Both crises should be handled within the forthcoming decades if not to develop beyond our control. Actions implemented to mitigate one challenge should not worsen the other, and solutions that address both at the same time are preferable. To some extent, the two crises are interlinked. Biodiversity, together with geophysical and climatic factors form and maintain ecosystems, which contribute to climate change mitigation by capturing CO2 and store carbon. On the other hand, the current climate change worsen the negative impact of the main drivers causing biodiversity loss, i.e., land use change, non-sustainable use of natural resources, invasive species and pollution. This leads to further degradation of ecosystems, which in turn may weaken the functionality of ecosystems that may reduce the ability of nature to capture and store carbon.
The United Nations sustainable development goals (SDGs) outline the urgent need to combat climate change and protect and restore biodiversity and ecosystems. The coming decade is declared The United Nations Decade on Ecosystem Restoration. Nature-based solutions are increasingly invoked to meet the range of objectives necessitated by restoration. Nature-based solutions however require informed decision-making and long-term planning. These context-specific approaches can offer transformative change but require stakeholders to learn from existing practices and develop consensus around best practices for a range of different situations. Key examples demonstrating positive outcomes for both biodiversity and climate can support larger scale objectives and policies.
Case studies
The project identified eight cases related to nature-based solutions enacted in the Nordic countries of Denmark, Finland, Iceland, Norway and Sweden. The report assess results and identifies potential synergies between biodiversity conservation and climate change mitigation.
Four case studies describe projects involving Nordic peatlands while the other four cases describe initiatives related to forests. Across the cases, synergies have been identified and defined as initiatives, which conserve and restore biological intactness of ecosystems and at the same time reduce the emission of greenhouse gases and/or accumulate and store carbon in these ecosystems.
Two peatland cases from Denmark and Sweden represent active and ongoing restoration of the hydrology and biological communities of degraded mires. A third case study from Norway examines the degradation of an entire peatland ecosystem, the Palsa mires. This unique habitat type faces the risk of complete extirpation due to climate change. The fourth peatland case from Finland describes research on future land-use of drained and afforested boreal peatlands no longer under commercial / economic valuation.
Two forest case-studies from Iceland and Norway examine the potential for restoring native deciduous forest ecosystems that have been cut down and converted primarily to agricultural use. Both studies focus on areas capable of natural or assisted regeneration in abandoned landscapes with minor human management. A third case from Denmark describes a government initiative to restore natural forests in Danish state lands. This case explores the process for designating future conservation areas to be abandoned by forest industry after initial restoration of conditions necessary for reintroducing biodiversity. A fourth case from Sweden presents research on boreal forests on how boreal forests accumulate organic carbon in tree biomass and soil. The development of biological communities, in long term undisturbed ecosystems of different age classes spanning several thousand years can enhance biodiversity and climate change mitigation goals.
Synergy
Tables 3.1 and 3.2 list synergies between biodiversity and climate mitigation objectives realized by the eight projects as well as literature sources that further describe these synergies.
Intact mires comprise a unique set of biological communities of varying diversity, which will be negatively affected if the biophysical and chemical conditions change. Peatlands moreover represent massive accumulations of organic carbon buried and stored in the form of soils that often extend several meters below the surface and have accumulated over thousands of years. Drainage of mires deteriorate biodiversity and cause emissions of CO2 from decomposition of the carbon stock. In terms of their climatic effects, these emissions carry the same risks and long-term costs to society as those produced by fossil fuel combustion.
Restoration of natural hydrology in drained peatlands typically produces immediate benefits in the form of an overall reduction in greenhouse gas emissions (expressed as the combined fluxes of CO2, CH4, N2O and DOC). Even in cases with substantial initial methane (CH4) emissions, the long-term climate benefits of rewetting drained peatlands (restoring natural water balance and flows) exceed those of maintaining drainage systems. Biodiversity will slowly recover in hydrologically restored mires, but the pace of recovery strongly depends on the degree of degradation and the new conditions.
For forests, consensus holds that natural undisturbed (primary) forests and other old growth forests host the most intact biodiversity. Thus, both conservation of such forests and nature restoration in previously managed forests benefit biodiversity. Conversely, forestry (commercially managed forest) entails negative impacts on biodiversity to varying degrees depending on the specific management practices. Forests influence climate both in terms of the carbon stock stored by the forest ecosystems in biomass and soil and through the uptake of CO2 from the atmosphere by photosynthesis in trees in particular. Thus, forests can mitigate climate change by maintaining the accumulated natural carbon stock or by increasing the stock (carbon sequestration) when younger trees and stands grow older.
Preservation and restoration of nature is essential for biodiversity and in combating climate change. Well-functioning ecosystems provides habitats for species, accumulate and store large amounts of carbon, and provide other ecosystem services such as recreational opportunities for humans (e.g., time spent in nature). The Convention on Biological Diversity, the Convention on Climate Change and the EU Biodiversity Strategy for 2030 list protecting and restoring peatlands and old growth forests as target objectives. These recently include allocating 30% of the land to nature protection by 2030 and phasing out net greenhouse gas emissions in all sectors by 2050. With their strong commitment to evidence-based approaches, high rates of educational attainment, economic resources, and willingness to collaborate in taking on grand challenges, the Nordic countries can not only achieve these goals but also provide leadership in demonstrating how to meet them. Monitoring and documenting progress towards targets and modelling adaptive approaches will be crucial in managing these global risks.
Policy options
The report concludes with ten policy options (see chapter 4 for details).
1. Restoring nature, as exemplified in this report, provides an excellent option for Nordic countries to align with and take lead in meeting international biodiversity and climate policy targets through nature-based solutions.
2. A stop for new drainage activities in mires is essential to preserve natural carbon stocks and conserve biodiversity.
3. Nordic countries can prevent emissions of carbon dioxide and initiate long-term biodiversity restoration by rewetting drained mires and peatlands including areas currently used for farming, forestry or peat excavation.
4. Strict protection of existing old growth forests by the exclusion of forestry will safeguard important biodiversity and contribute to climate change mitigation through the preservation of natural carbon stocks.
5. Restoration of forest ecosystems allowing them to develop towards natural old growth forests is essential to biodiversity conservation and contributes to climate change mitigation and ecosystem resilience.
6. Conservation actions in managed forests can be a supplementary option, which is significantly less effective for preserving biodiversity, but with the potential to obtain or retain the possible climate benefits and economic return from forestry.
7. Improved documentation of the greenhouse gas dynamics and biodiversity of intact and restored ecosystems, preferably at the same location, is essential to develop informed and efficient nature based solutions and strategies.
8. Planning at large spatial scales will increase the efficiency and facilitate both local and overall synergies between biodiversity conservation and climate change mitigation.
9. Enhanced national mechanisms to provide advice and communicate results of scientific research on biodiversity and climate change will improve decision-making and public debate
10. Ambitious cross-sectorial and cross-disciplinary policies can facilitate the wider use of cost-efficient nature-based solutions to meet the biodiversity and climate challenges.
List of Acronyms
| CAP | EU Agricultural Policy |
| CBD | Convention on Biological Diversity |
| CH4 | The chemical formulae for methane |
| CO2 | The chemical formulae for carbon dioxide |
| DOC | Dissolved Organic Carbon |
| EU | European Union |
| GHG | Greenhouse gas |
| GWP | Global Warming Potential |
| IPBES | Intergovernmental Platform on Biodiversity and Ecosystem Services |
| IPCC | Intergovernmental Panel on Climate Change |
| IUCN | International Union for Nature Conservation |
| LBII | Local Biodiversity Intact Index |
| LULUCF | Land Use and Land Use Change and Forestry |
| NDC | Nationally Determined Contribution |
| NorBalWet | Nordic Baltic Wetlands Regional Ramsar Initiative |
| N2O | The chemical formulae for nitrous oxide |
| PREDICTS | Projecting Responses of Ecological Diversity In Changing Terrestrial Systems |
| RIS | Ramsar Information Sheet |
| SDG | Sustainable Development Goal |
| UN | United Nations |
| UNEP | United Nations Environment Programme |
1. Introduction
We face two interrelated global crises the loss of biodiversity and climate change (IPBES 2019, IPCC 2019). The UN’s sustainable development goal (SDG; https://sdgs.un.org/goals) no. 13 addresses the urgent need to arrest and manage climate change. SDG no. 15 seeks to protect biodiversity and restore ecosystems on land (see also UNEP 2019). World leaders including those representing Nordic countries have committed to these goals. Biodiversity and climate change are interdependent phenomena whose myriad connections are often scientifically conceptualized as feedback systems. On the one hand, species form ecosystems that perform natural carbon sequestration, cycle atmospheric gases, and form the base of the food chain through primary productivity. On the other, climate change in the form of rising temperatures and shifting weather patterns on larger times scales can disrupt food sources and destabilize habitats. These pervasive and large-scale impacts of climate change are a major driver of biodiversity loss after land use and direct exploitation of species (IPBES 2019). Nature-based solutions to preserve biodiversity can also mitigate climate change primarily through enhanced carbon storage but also through uptake over time.
Approaches to managing changes in precipitation, severe storms, and sea level rise can also involve nature-based solutions that enhance biodiversity (e.g., restoration of coastal dunes or other habitats) or adaptation to situations of increased flooding etc. While broader integrated approaches or adaptation measures are critical examples of synergies, they lie outside the scope of this report.
Nature-based solutions are typically implemented at national and local levels. Bodies and institutions operating at these levels will make decisions and carry out specific initiatives. Cohesive action requires instantiation on political grounds and geographical scales. These problem spaces have so far proved intractable in terms of meeting biodiversity targets over the last two decades (IPBES 2019). Missed opportunities have produced an even more urgent need to demonstrate by example that targets can be met. Specific cases of success or positive developments for biodiversity and climate will encourage more and coordinated action.
The concept of nature-based solutions is still under development. The concept has been put forward by practitioners (in particular the International Union for Nature Conservation, IUCN) and quickly thereafter by policy (European Commission), referring to the sustainable use of nature in solving societal challenges. The definition by IUCN is below (see also Eggermont et al. 2015).
Nature-based solutions as defined by IUCN 2021
Nature-based Solutions are actions to protect, sustainably manage and restore natural and modified ecosystems in ways that address societal challenges effectively and adaptively, to provide both human well-being and biodiversity benefits. They are underpinned by benefits that flow from healthy ecosystems and target major challenges like climate change, disaster risk reduction, food and water security, health and are critical to economic development. https://www.iucn.org/theme/nature-based-solutions/about
Recent climate and biodiversity reports have emphasized the importance of local action (IPBES 2019, IPCC 2019) but national level leadership is also critical. Among other nations, Nordic governments not only acknowledge the risks of biodiversity loss and climate change they also possess the imperatives to address these issues with effective action. Factors contributing to consensus on reducing risk arise from a societal tradition of evidence-based approaches, high educational attainment levels, strong cultural and social institutions, and adequate economic resources.
Peatlands and forests are particularly important for both biodiversity and climate regulation due to their ability to sequester and store carbon and for their biodiversity. These eight cases take departure in either of these two ecosystems, which in some cases overlap. The project promotes the use of knowledge on synergetic solutions, and it will hopefully inspire and support Nordic leadership at the interface between biodiversity conservation and climate change mitigation including for example the use of nature-based solutions feeding into nationally determined contributions (see also IUCN 2019).
2. Background
2.1. Biodiversity and Climate Change in the Nordic Region
Peatlands and forests are shaped by climate and are important for regulation of climate because they uptake and store carbon. Peatlands represent one of the largest terrestrial reservoirs of carbon on Earth and have residence times of hundreds or thousands of years (see below). Carbon is also stored in the biomass of trees and some peatland soils are covered by trees and the two ecosystems overlap in time and space.
The number and abundance of species and both genetic and ecosystem diversity have increased in Nordic regions since the last ice age. This can be attributed to natural factors such as the gradually immigration of plants and animals into the barren land after the ice (Hallanaro & Pylvänäinen 2001).
The region is rich in biodiversity and spans from temperate regions in the south over the boreal region to tundra in the arctic regions in the north and thus represents an immense range in climate and geophysical parameters. However, in a global perspective the Nordic region generally hosts more widespread species and a lower biodiversity compared to most tropical regions. Never-the-less Nordic biodiversity is important in our daily lives and as a contribution to overall global biodiversity. The human effect on biodiversity and landscapes have increased dramatically over the last several thousand years and especially during the last couple of centuries human driven biodiversity loss has taken place in the Nordic region (Hallanaro & Pylvänäinen 2001) as well as globally (IPBES 2019) both in genetic diversity as well as species and ecosystem level (see e.g. cases presented here).
Nordic biodiversity has been described in a number of publications for example Hallanaro & Pylvänäinen (2001). Here the focus is on two widespread Nordic ecosystems namely peatlands and forests. IPBES (2018) describes the global loss of biodiversity and soil organic carbon. Nordic research in the areas of land degradation and restoration can therefore inform approaches to large-scale problems.
2.1.1. Peatlands
Peatlands are wetlands with a high content of organic carbon. Mires are peatlands with a living biological community accumulating peat. Mires host native Nordic biological communities, which include a number of unique species and species interactions. Peatland forms in areas of excessive moisture where waterlogged conditions prevent the complete decomposition of plant material. The distribution and character of peatlands therefore strongly depends on climate (Barthelmes et al. 2015). Tab. 2.1 lists estimates of mire and peatland area among Nordic countries (Joosten et al. 2017).
| Country | Mire area km2 | Peat area km2 | Country area km2 | Criteria |
| Denmark | 137 | 2,029 | 43,000 | |
| Finland | 35,000 | 90,000 | 338,000 | > 0 cm peat |
| Iceland | 2,112 | 5,777 | 103,000 | > 12% organic carbon |
| Norway | 37,700 | 44,700 | 324,000 | |
| Sweden | 52,300 | 63,700–69,200 | 450,000 |
Tab. 2.1. The area of mire (peatland with actively growing natural vegetation) and of total peatland area (including degraded organic soil) in Nordic countries. Differences primarily reflect differences in total land area and the degree of drainage conducted in the respective countries. Source: Joosten et al. 2017.
Peatlands are found throughout the Nordic region. In the subarctic and arctic zone peatlands are influenced by permafrost and polygon and palsa mires comprise peatland types influenced by permafrost. In the boreal zone peatlands cover vast areas comprising Aapa mires in areas with a positive water balance where cool conditions limit evapotranspiration including in areas with low precipitation (Barthelmes et al. 2015). In the temperate zone in south peatlands are found as raised bog and fens in areas with exceeding rainfall and in basins with ground water flow (see below).
Mires are often classified as ombrotrophic and minerotrophic mires depending on whether they receive their nutrients and water from rain or from streaming or ground water (Joosten in press.). Thus, in ombrotrophic mires the peat layer is thick and have often been accumulated for hundreds or thousands of years and are fed by rain. These mires are acid and nutrient poor and usually covered by a thick layer of Sphagnum mosses usually known as bogs including raised bogs and blanket bogs. In the minerotrophic mires the layer of peat is usually thinner and they are fed by streaming water and/or groundwater to various degrees as well as precipitation (Fig. 2.1) and known as fens. A great variety of intermediate mires exists and they are also influenced by nutrients and minerals, e.g. rich or poor fens depending on the classifications.
The plant community is often used to define the mire types. A thorough assessment of this diversity based on vegetation and characteristic species in Europe is presented in (Joosten et al. 2017), which include country chapters for all the Nordic countries. Mires are generally species poor compared to for example forests, but they nevertheless host unique and protected biological communities (see also chapter 2.3. on policy agreements).

Fig. 2.1. Schematic diagrams of different types of bogs and fens. Bogs receive nutrients from the air while fens are largely fed by ground water. Transitional mires are largely influenced by rainfall but can also receive ground water input. Source: Joosten (in press., Ramsar Convention on Wetlands).
Various species of Sphagnum mosses usually predominate in Nordic mires. These mosses cover the surface of many mires while dead plant material covered by water below surface gradually transitions into peat. Sphagnum is important because their cells are capable of absorbing large amounts of water and thereby contribute to keep a high-water table (Joosten in press.). Few vascular plants live in mires (there are exceptions) due to the acidic and often nutrient poor conditions. Invertebrates include e.g. nematodes, mites, spiders, ants, beetles and other insects. Mires also play an important in the life cycles of aquatic insects such as mosquitos, horse-flies, and black flies (Hallanaro & Pylvänäinen 2001). Their considerable biomass attract e.g. insect-eating birds.
Peatlands play an important role in global climate regulation and constitute the largest terrestrial store of carbon (Parish et al. 2008, Barthelmes et al. 2015). Mires act generally as net carbon sink removing CO2 from the atmosphere by photosynthesis while at the same time emitting CH4. However, in the long-term accumulation of carbon takes place. Drainage of mires releases large amounts of CO2 and sometimes N2O (Barthelmes et al. 2015) that act as greenhouse gasses (GHGs).
The large carbon stock is comprised of layers of peat under waterlogged conditions accumulated over centuries or millennia (Fig. 2.2). Most originated at the onset of the Holocene and have accumulated peat and thereby bound large amounts of carbon from the atmosphere for the past 10,000 years.

Fig. 2.2. Cross section of a mire (raised bog) showing the peat column. b) Peat accumulation over time, and c) net gain of peat showing litter (dead vegetation) accumulation in excess of decomposition. Illustration from Page & Baird (2016) in Bartlett et al. 2020.
In addition to climate, hydrological and hydro-chemical factors determine rates of carbon accumulation in peatlands. Nutrient rich fens typically exhibit higher rates of accumulation. The rates generally increase in nutrient rich fens and decrease with nutrient poor conditions.
In peatlands the dead plant material is subject to aerobic decay only for a limited time because it soon arrives in a permanently waterlogged and oxygen poor environment where the rate of decay is an order of magnitude lower (Barthelmes et al. 2015). Thus, a high-water table is essential for climate regulation because lowering the water table results in decomposition of the plant material due to access of oxygen and the release of CO2 and N2O turning drained peatlands into carbon sources. Peatlands are roughly GHG neutral when the mean water table level is in the range of 0 to 10 cm below the surface (Barthelmes et al. 2015, Bartlett et al. 2020) and become emitters when inundated due to release of CH4.
In Nordic regions, large areas of peatland have been drained primarily for agriculture, forestry, or peat extraction. In the region as a whole, about 44% of the peatlands have been drained. This exceeds the global average of 12% but falls below the percentage of degraded peatlands estimated for the whole of Europe of 60%. However, there are large variations among the Nordic countries: Denmark has drained about 93% of its peatlands, Finland has drained 78%, Iceland 63%, Sweden 18% and Norway 9% (Barthelmes et al. 2015).
The climate burden of degraded peatlands
Whereas natural peatlands have been cooling the global climate over the last 10,000 years, drained and degraded peatlands are powerful sources of carbon dioxide (CO2) and nitrous oxide (N2O). These greenhouse gases (GHGs) result from microbial oxidation of organic matter when air penetrates the formerly water-saturated peat. The drier conditions following drainage also increase the risk of fire (Kettridge et al. 2015, Sirin et al. 2020).
The emissions from peatland exploitation, degradation and fires are currently responsible for some 5% of global anthropogenic GHG emissions. Continuing emissions from drained peatlands until 2100 may comprise 12–41% of the remaining GHG emission budget for keeping global warming below +1.5 to +2 °C (Leifeld et al. 2019). Further scenario studies indicate that the global land sector will by 2100 be a net carbon source, unless all presently intact peatlands remain intact and at least 60% of the currently degraded peatlands are in the coming decades rewetted (Humpenöder et al. 2020). This implies, that with rewetting ‘only’ 60% of the degraded peatlands, the carbon sink capacity of the entire remaining land sector (af- and reforestation, improved forest management, carbon sequestration in mineral soils) will merely serve to compensate for the carbon losses from the remaining 40% of degraded peatlands and will not contribute to the ‘net carbon sinks’ (cf. IPCC 2018).
Modified after Joosten (in press.).
2.1.2. Forests
Forest once covered most of the Nordic region south of the arctic and below tree lines. Today, more than 650,000 km2 of forest cover more than half of the land area of Sweden and Finland and more than a third of the land area of Norway (Framstad et al. 2013). However, most Nordic forest has been subject to major human alteration beginning in southerly regions approximately 6000 years ago (Berglund 1991, Myhre & Øye 2002; both cited in Framstad et al. 2013 and Fritzbøger & Odgaard 2017). These impacts include logging for wood or agricultural clearing such that few old growth or primary forests remain. Denmark and especially Iceland have been virtually entirely deforested. Today, Denmark has restored the forest cover of about 15% of its land area (about 6000 km2 Nord-Larsen et al. 2019) while Iceland’s forest cover is still only a few percent or about 1500 km2 (Snorrason et al. 2016).
Most Nordic forests categorized as boreal coniferous forests (taiga) dominated by the native species Scots pine (Pinus sylvestris) and Norway spruce (Picea abies) (Hallanaro & Pülvänäinen 2001). Temperate, or “nemoral”, broadleaved deciduous forests occur scattered throughout southern Norway and occur more widely distributed in southern Sweden. These also formed the predominant native forest types of Denmark. Common native tree species include the Beech (Fagus sylvatica), the Oaks (Quercus robur) and (Q. petraea), the Ash (Fraxinus excelsior), the Maple, (Acer platanoides), and the Lime (Tilia) spp. (Hallanaro and Pülvänäinen 2016). Mountain birch forms a unique type of forest in the northernmost regions between the boreal coniferous forest and the arctic tundra (Hallanaro and Pülvänäinen 2016). These forests host a unique variety of Downy birch, (Betula pubescens), and represent the only native woodland and scrub habitats found in Iceland (e.g., Aradottir et al. 2013).
Vast and variegated natural forests formed by climatic, geological and biological factors occupied the Nordic region over hundreds of thousands of years between Pleistocene ice ages. These natural forests thus comprise the habitats to which most Nordic species are evolutionarily adapted. However, apart from the mountain birch woodlands, the vast majority of forests found in Nordic regions today, are typically managed for wood production (e.g., Framstad et al. 2013). Forest management, including widespread clear-cutting, has significantly reduced the natural biodiversity.
Forest management entails reduction and alteration of many specific habitat types found in natural undisturbed forests. In managed forests, trees in each stand are typically uniform in terms of species, size, and age. Understory vegetation is actively removed. Old trees and deadwood are missing because trees are typically harvested at a biologically young age. Clearcutting is common practice (removing all trees at once) particularly in coniferous forests. Management practices also include soil treatment prior to planting the next generation of trees. Together these practices represent major disturbances, which are not comparable to the dynamics of natural forest ecosystems. Drainage of moist and wet soils to promote tree growth are also widespread, and in some regions, fertilizer is applied. All these practices substantially reduce and alter the natural heterogeneity and dynamics that create habitats and sustain biodiversity in natural forests (e.g., Berg et al. 1994, Christensen & Emborg 1996, Paillet et al. 2010, Müller & Bütler 2010, Rudolphi & Gustafsson 2011, Lelli et al. 2018).
Nordic forests not only sustain regional biodiversity, they also regulate climate. Forest ecosystems perform a number of fluxes in the terrestrial carbon cycle including absorbing and storing major fractions of carbon. Trees take up CO2 and convert it into carbon stored in standing trees, understory vegetation, litter, dead wood, and soil. Therefore, forest ecosystems represent some of the most important natural carbon sinks and carbon stores, both globally and in the Nordic region. Fig. 2.3 shows typical forest carbon cycle processes. Different types of forests behave differently in terms of carbon sinks and fluxes. In addition to climatic conditions, soil type, and forest age, the dominant tree species also influence carbon cycle dynamics. Young forests accumulate carbon faster than old forests but old forests hold larger stocks of carbon.
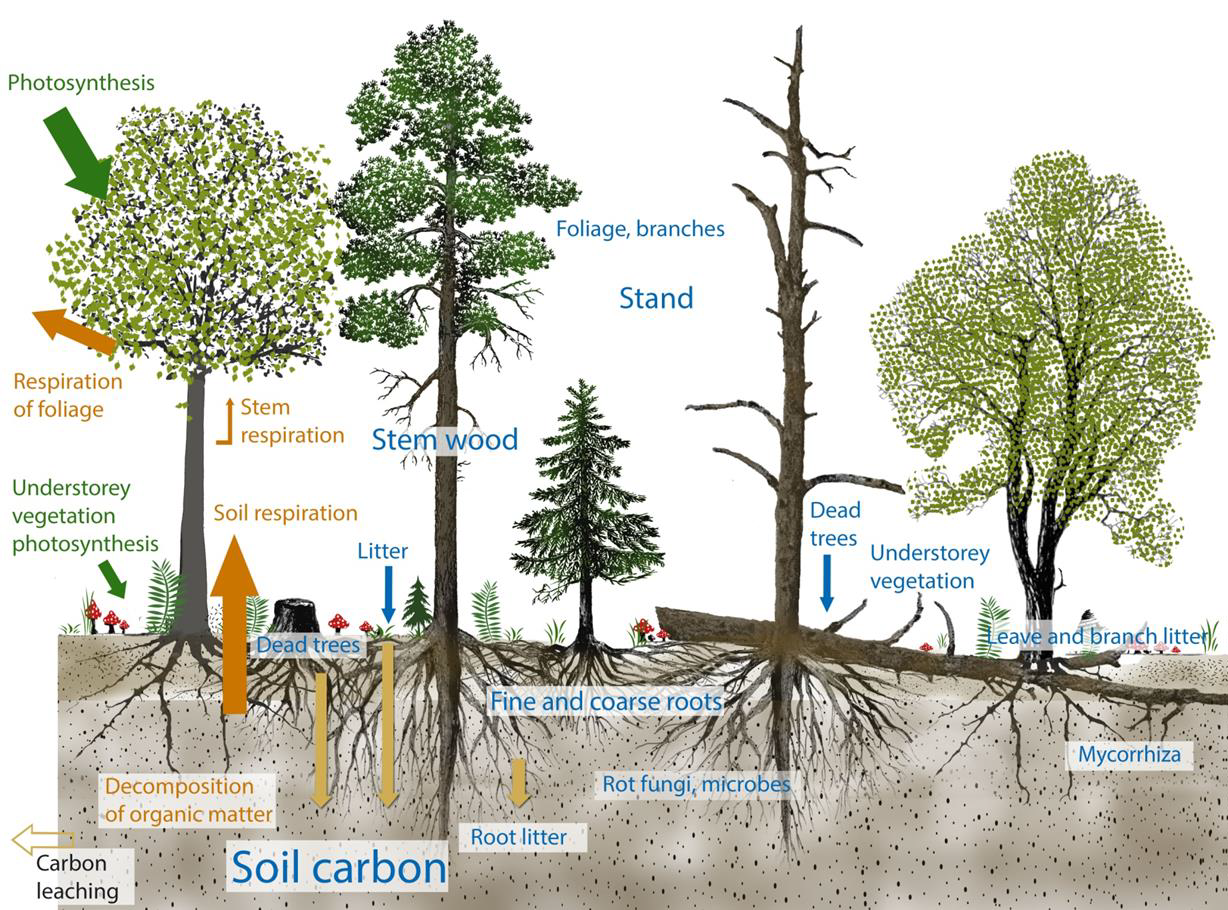
Fig. 2.3. The carbon cycle of the forest ecosystem. Source: Onarheim (2018, Mires and Peat, 24, art. 27).
Commercial forestry, including logging, soil treatment, and drainage, strongly influences the carbon cycle and carbon stocks stored by forests. The carbon stock of a managed forest is usually smaller than that found in natural old growth forests because trees are smaller due to frequent harvest (Harmon et al. 1990, Ciais et al. 2008, Mäkipää et al. 2011). Furthermore, understorey vegetation and dead wood are kept at lower proportions. Harvesting especially by clearcutting often reduces the soil carbon stock (e.g., Peltoniemi et al. 2004, Häkkinen et al. 2011). Drainage also reduces soil carbon and leads to higher CO2 emissions from the forest due to higher rates of decomposition of accumulated organic material like peat (e.g., Ojanen & Minkinen 2019, IPCC 2014). Some circumstances mitigate the climatic impacts of managed forests. Harvested wood products from managed forests contribute to climate mitigation by substitution of fossil fuels and building materials that require more fossil fouel for their production, and through carbon storage in wood products (Sathre et al. 2010, Leskinen et al. 2018). However, researchers and policy makers continue to debate the relative merits of forest conservation versus management for wood production in terms of their respective climatic impacts (e.g., Searchinger et al. 2018, Taeroe et al. 2017, Nabuurs et al. 2017).
Overall, protection of existing old growth forests and restoration of natural forest ecosystem represents important opportunities for conservation of biodiversity and mitigation of climate change. A thorough assessment of the “Biodiversity, carbon storage and dynamics of old Northern forests” with focus on the Nordic region is given in Framstad et al. (2013). Fig. 2.4. gives an overview of greenhouse gas fluxes in a forest ecosystem.

Fig. 2.4. Mass transfer components and CO2, CH4, and N2O fluxes contributing to soil carbon stock and fluxes in a forest ecosystem subject to drainage as in IPCC (2014). Source: Jauhiainen et al. (2019).
2.2. Approach and methodological considerations
The identification and description of eight case studies to illustrate potential synergy between biodiversity conservation and climate change mitigation is the primary aim of this report. Moreover, focus was limited to a few key ecosystems in order to bring forward related cases, and thereby consolidate findings and provide more robust policy advice. A focus on wetlands and forests was chosen because both ecosystems constitute important natural carbons stocks and host important and threatened biodiversity. Thus, a focus on only two main ecosystem types was prioritized over including e.g. marine ecosystems (“blue carbon”) such as seagrass beds or other wetland types. Finally, our focus is mainly on climate change mitigation rather than adaptation.
The specific case studies were selected based on criteria, which included the need for evidence from scientific research, monitoring or other relevant studies and ideally on both biodiversity and climate at the same time. Moreover, an opportunity to learn from the case was essential. Thus, there should be a story and information from the case to share of general Nordic interest. Finally, a geographical balance within the Nordic region is needed to cover a range of different habitats and contexts within the two selected ecosystems. Eventually, four peatland and four forest cases were identified in five Nordic countries: Denmark, Iceland, Finland, Norway and Sweden. For all cases, key persons in the respective countries were contacted and meetings were held with scientific researchers and government officials. The relevant persons are mentioned in the acknowledgement sections in each case study.
It has been a general challenge to identify cases with substantial evidence on both climate and biodiversity. With a few exceptions, more comprehensive studies were conducted within one of these two fields. To meet the need for national cases of great regional interest, cases at very different geographical scales were included, ranging from just one locality in one case to restoration of ecosystems at national or even regional level in other cases.
2.2.1. Biodiversity
One of the definitions of biodiversity used by IPBES (2020) is:
The variability among living organisms from all sources including terrestrial, marine and other aquatic ecosystems and the ecological complexes of which they are a part. This includes variation in genetic, phenotypic, phylogenetic, and functional attributes, as well as changes in abundance and distribution over time and space within and among species, biological communities and ecosystems.
This is a slight change from the definition used by the Convention on Biological Diversity (CBD), which was defined twenty years earlier:
“Biological diversity" means the variability among living organisms from all sources including, inter alia, terrestrial, marine and other aquatic ecosystems and the ecological complexes of which they are part; this includes diversity within species, between species and of ecosystems.
Thus, a stronger focus on variations in time and space is introduced as well as variation at the genetic level and of functional attributes and generally a somewhat more dynamic concept.
It is difficult to provide an all-encompassing measure of biodiversity in the field situation and in that regard, biodiversity is different from measuring climate change. However, our overriding assumption is that intact ecosystems are important for biodiversity as well as for functions and processes. Moreover, it is our assumption that intact ecosystems are important for biodiversity at the three levels of its definition i.e. for genetic diversity, for species diversity (including abundance) and for ecosystem diversity.
In this report we primarily focus on biodiversity at habitat and ecosystem level, although assessment of indicator species is included as well in some cases. We have a focus on native biological communities including their functions and biological processes, which primarily have developed in our region after the last ice age. It has been the aim to focus on functional ecosystems and habitats supporting biological processes and interactions and, in that way, promote individual species and biological communities and thereby, also genetic diversity.
One emerging field in this regard is the work with biological intactness in relation to what has been lost due to human impact (Scholes & Biggs 2005, Newbold et al. 2015), which at the moment use species as surrogates for biodiversity. Newbold et al. (2015) states: Human activities, especially conversion and degradation of habitats, are causing global biodiversity declines. How local ecological assemblages are responding is less clear—a concern given their importance for many ecosystem functions and services. These attempts have recently turned their focus into local diversity and move away from global measures such as the total species extinction because resilience of ecosystem functions and services are likely to depend on local diversity (Newbold et al. 2015).
The models by Newbold et al. (2015) suggested that land-use changes and associated pressures strongly reduce local terrestrial biodiversity e.g. in peatlands and forests and including species richness at different scales and total abundance (Newbold et al. 2015, see also IPBES 2019). In other words, such work moves towards estimating how much of a terrestrial site's original biodiversity remains. These assessments are based on a specific “Local Biodiversity Intactness Index” (LBII), for background information see Newbold et al. (2015), Hudson et al. (2016): https://www.predicts.org.uk/pages/policy.html .
Because the local biodiversity intactness index (LBII) relates to site-level biodiversity, it can be averaged and reported for any larger spatial scale (e.g., countries, biodiversity hotspots or biomes as well as globally) without additional assumptions. However, they still rely to a large degree on species richness data in a database: PREDICT (Hudson et al. 2014).
We have used the general assumption that intact ecosystems with an original assemblage of species and their interactions comprise a baseline where biological functions and processes are preserved. We have used the general assumption that the preindustrial state of an ecosystem would seem to be the most ideal reference condition, and that this could be approximated in practice by contemporary data from minimally impacted sites (see e.g. Purvis et al. 2018) and acknowledging that ecosystems with its assemblages of biological communities are constantly evolving and not a steady state.
There is no comprehensive or complete biodiversity studies in any of the Nordic cases presented in this report. Biodiversity intactness is still an emerging biological discipline, which underlines the challenge of identifying a generally accepted “currency” for measuring biodiversity, which is also relevant at the local level.
Moreover, a common aim in recent biodiversity management is the move towards restoring (or conserving existing) self-regulatory habitats and ecosystems with a need for limited or no human interventions (Barfod et al. 2020 as an example from Denmark). This will often require larger areas set aside for nature.
2.2.2 Climate
Research in carbon cycles including flux and emissions of greenhouse gas is perhaps one of the most rapid developing research fields at the global level. Measuring fluxes at local sites (e.g. An & Zong 2016) or presenting regional or global reviews is developing fast. Nevertheless, it has been difficult to find cases in the Nordic countries with comprehensive field studies involving greenhouse gas flux monitoring and carbon stock measures, which also has comprehensive information on biodiversity (see cases chapter 6).
Certainly, one of the most important reasons for peatland rewetting and restoration is climate change mitigation (Griscom et al. 2017). Although peatland restoration initially has been targeted biodiversity restoration in the Nordic region, the climate benefits from certain biodiversity restoration projects have become increasingly clear within the last decade (e.g. Barthelmes et al. 2015). This is because the huge emissions of greenhouse gasses from drained peatlands can be significantly reduced by raising the water levels closer to the ground level. The more exact level of the water table needed to have the maximum effect depends on the specific context and the peatland type (Joosten in press.).
The water level is key to the climate impact from a peatland. In the drained situation, there will be significant greenhouse gas emissions. The deeper the water table, the larger the emissions (Joosten in press.). With water table at the ground – the case in many natural peatlands – the balance between different greenhouse gasses becomes more delicate. With a high water table – as in the natural state – there is an accumulation of organic material over time and the emission of CO2 is almost zero. In an intact mire or a rewetted peatland, the accumulated plant material (peat) is decomposed anaerobically (without oxygen) resulting in the emission of CH4, a greenhouse gas 28 times stronger than CO2. However, in general, rewetting of drained peatlands instantly leads to benefits: The overall greenhouse gas effect (expressed as the combined fluxes of CO2, CH4, N2O and DOC) becomes positive for the climate, compared to the former drained situation and the carbon sink function is being restored (Nugent et al. 2018, Joosten in press.).
Even in case of a large initial methane peak, the longer-term climate effects of rewetting are better than maintaining the drained status quo. The reason is that CH4 has a shorter atmospheric lifetime compared to CO2 and N2O, which actually accumulate in the atmosphere, whereas the atmospheric concentrations of CH4 quickly reach a steady state (Joosten in press., Fig. 2.5.).


Fig. 2.5.: Radiative forcing (RF) and global climatic warming effects (relative to 2005) of peatland management without (left) and with (right) an initial 10 times larger methane peak for 5 years after rewetting. Drain_More: The area of drained peatland continues to increase from 2020 to 2100 at the same rate as between 1990 and 2017; No_Change: The area of drained peatland remains at the 2018 level; Rewet_All_Now: All drained peatlands are rewetted in the period 2020–2040; Rewet_Half_Now: Half of all drained peatlands are rewetted in the period 2020–2040; Rewet_All_Later: All drained peatlands are rewetted in the period 2050–2070. Source: Günther et al. (2020). Nature Communications 11:1644.
Global Warming Potential
Global warming potential (GWP) is a relative measure of how much heat a greenhouse gas traps in the atmosphere. It compares the amount of heat trapped by a certain mass of the gas in question to the amount of heat trapped by a similar mass of carbon dioxide. GWP is calculated over a specific time interval, commonly 20, 100 or 500 years. GWP is expressed as CO2-equivalents (CO2-e) i.e. as a factor of carbon dioxide (whose GWP is standardized to 1). Source: Barthemeles et al. (2015).
Often, there is no specific flux measurements of GHG at a site or in a given habitat. In this case an assessment may rely on standard emission factors from the IPCC guidance (IPCC Wetlands Supplement 2014) applying the use of standard values using the IPCC guidelines on emissions (2006). However, these standard figures are generally intended for indicative national inventories only, and not for detailed locality specific studies, which means that there is a large degree of uncertainty in these calculations. Site-specific measurements are preferable.
2.3. International policy agreements
A number of international policy agreements are relevant for the policy options provided in this report (chapter 4).
2.3.1. Sustainable Development Goals (SDGs)
Sustainable Development Goals (SDGs) are 17 interlinked global goals set in 2015 by the United Nations General Assembly. The three Sustainable Development Goals (SDGs) of particular relevance for biodiversity and climate in peatlands and forests are stated below. These goals come with a set of related targets and indicators specifying the goals into more concrete measurable actions and the most relevant are indicated below. The timeline for some targets has unrealistically been set to 2020, as these follow the Aichi Targets to be met in 2020, however, as they have not been met (IPBES 2019), activities for their fulfillment must be expected to continue for the time being. New targets are under development as part of the Post Biodiversity Framework (CBD 2020).
Goal 15 - Life on Land: Protect, restore and promote sustainable use of terrestrial ecosystems, sustainably manage forests, combat desertification, and halt and reverse land degradation and halt biodiversity loss.
Target 15.1: By 2020, ensure the conservation, restoration and sustainable use of terrestrial and inland freshwater ecosystems and their services, in particular forests, wetlands, mountains and drylands, in line with obligations under international agreements. Indicator 15.1.1: Forest area as a proportion of total land area. Indicator 15.1.2: Proportion of important sites for terrestrial and freshwater biodiversity that are covered by areas, by ecosystem type.
Target 15.2: By 2020, promote the implementation of sustainable management of all types of forests, halt deforestation, restore degraded forests and substantially increase afforestation and reforestation globally. Indicator 15.2.1: Progress towards sustainable forest management.
Target 15.5: Take urgent and significant action to reduce the degradation of natural habitats, halt the loss of biodiversity and, by 2020, protect and prevent the extinction of threatened species. Indicator 15.5.1: Redlist index.
Goal 13 - Climate action: Take urgent action to combat climate change and its impacts.
Target 13.2: Integrate climate change measures into national policies, strategies and planning. Indicator 13.2.1: Number of countries that have communicated the establishment or operationalization of an integrated policy/strategy/plan which increases their ability to the adverse impacts of climate change, and foster climate resilience and low greenhouse gas emissions development in a manner that does not threaten food production (including a national adaptation plan, nationally determined contributions, nationally communication, biennial update report or other).
Goal 6 – Clean water: Ensure availability and sustainable management of water and sanitation for all
Target 6.5: By 2030, implement integrated water resources management at all levels, including through transboundary cooperation as appropriate. Indicator 6.5.1: Degree of integrated water resources management implementation (0–100)
Target 6.6: By 2020, protect and restore water-related ecosystems, including mountains, forests, wetlands, rivers, aquifers and lakes. Indicator 6.6.1: Change in the extent of water-related ecosystems over time
Moreover, peatlands and forest ecosystem restoration providing synergy between biodiversity conservation and climate change mitigation provides for related services and benefits supporting various of the other interdependent sustainable development goals and targets.
2.3.2. The Conventions on Biodiversity and Climate Change
The Convention on Biological Diversity and the post 2020 biodiversity targets are under development and presently in draft form, however, the COVID-19 has postponed the COP15, which was supposed to agree on biodiversity targets for the next decade. The EU has a vision of taking a leading role in the development of the post 2020 biodiversity agenda hence the EU biodiversity strategy (June 2020) is of relevance in this regard (see below).
The Aichi Targets for biodiversity set for 2020 have unfortunately not been met. A thorough assessment was conducted as part of the global report from IPBES (2019) concluding that it was unlikely to meet most of these targets. They included globally agreed targets relevant for biodiversity in peatlands and forests including for example:
Target 5: The rate of loss of all natural habitats, including forests, is at least halved and where feasible brought close to zero, and degradation and fragmentation is significantly reduced.
Target 11: At least 17% of terrestrial and inland water, and 10% of coastal and marine areas, especially areas of particular importance for biodiversity and ecosystem services, are conserved through effectively and equitably managed, ecologically representative and well-connected systems of protected areas and other effective area-based conservation measures, and integrated into the wider landscapes and seascapes.
Target 12: The extinction of known threatened species has been prevented and their conservation status, particularly of those most in decline, has been improved and sustained.
Target 15: Ecosystem resilience and the contribution of biodiversity to carbon stock have been enhanced, through conservation and restoration, including restoration of at least 15% of degraded ecosystems, thereby contributing to climate change, mitigation and adaptation and to combat desertification.
The Paris agreement from 2015 under the convention sets the target in 2050 of no net emission of greenhouse gasses and with the aim to limit global warming to 1.5 to 2 degrees C above pre-industrial levels. The Paris Agreement enables countries to deliver on their national climate action plans under the Paris Agreement (“Nationally Determined Contributions”, or “NDCs”), thereby promoting further ambitions to tackle climate change over time.
Nationally determined contributions (NDCs) are at the heart of the Paris Agreement and the achievement of these long-term goals. NDCs embody efforts by each country to reduce national emissions and adapt to the impacts of climate change. All Parties are requested to submit the next round of NDCs (new NDCs or updated NDCs) by 2020 and every five years thereafter.
For the land-use, land-use change and forestry sector, emissions and removals the following reporting categories are included: forest land, cropland, grassland, and wetland (wetland remaining wetland only from 2016), including land use changes between the categories, and between these categories and settlements and other land. The five carbon pools above-ground biomass, below-ground biomass, litter, dead wood and soil organic matters are included. In addition, the carbon pool harvested wood products is included.
The current submission is based on the IPCC Guidelines 2006 combined with the emission factors from the 2013 Wetlands Supplement (IPCC 2014) Chapter 2 and 3 for CO2, N2O and CH4 combined with national derived emission factors. The LULUCF sector differs from the other sectors in that it contains both sources and sinks of carbon dioxide.
2.3.3. Other global agreements and science-policy platforms
IPCC i.e. the Intergovernmental Panel on Climate Change was created as an independent scientific body to provide policymakers with regular scientific assessments on climate change, its implications and potential future risks, as well as to put forward adaptation and mitigation options.
IPCC 2019:
While some response options have immediate impacts, others take decades to deliver measurable results. Examples of response options with immediate impacts include the conservation of high-carbon ecosystems such as peatlands, wetlands, rangelands, mangroves and forests. Examples that provide multiple ecosystem services and functions, but take more time to deliver, include afforestation and reforestation as well as the restoration of high-carbon ecosystems, agroforestry, and the reclamation of degraded soils
A wide range of adaptation and mitigation responses, e.g., preserving and restoring natural ecosystems such as peatland, coastal lands and forests, biodiversity conservation, reducing competition for land, fire management, soil management, and most risk management options (e.g., use of local seeds, disaster risk management, risk sharing instruments) have the potential to make positive contributions to sustainable development, enhancement of ecosystem functions and services and other societal goals
IPBES i.e. the Intergovernmental platform on biodiversity and ecosystem services provides global and regional assessments to strengthen the science-policy interface and provide scientific information on biodiversity and related ecosystem services (Natures Contribution to People) including climate regulation for the conservation and sustainable use of biodiversity, long-term human well-being and sustainable development. Eight comprehensive assessment reports have so far been produced reviewing thousands of papers related to biodiversity including an assessment of the before mentioned Aichi Targets.
The reports states that biodiversity loss is accelerating and that we are degrading land in terms of for example loss of organic carbon soil and native species and that less than one quarter of the land surface is now wilderness with intact ecological and evolutionary processes. However, one of the key findings in the reports is that it is still possible to change the course but a transformative change is needed if we are going to succeed in bending the curve of biodiversity loss as has been global targets to be met since 2010. This implies according to IPBES (2019) changes in all dimensions of life including changing economic, social, political and technological factors.
A number of other relevant conventions exist for example the Convention on Wetlands (Ramsar), which has been instrumental in the conservation and wise use of peatlands. The Nordic countries have played a key role in this regard through common statements and various resolutions on peatlands through the Nordic Baltic Wetlands cooperation – a regional initiative under the Ramsar Convention comprising the Nordic and the Baltic states. Moreover, the history and recognition of peatlands as important for biodiversity and climate is apparent from the historical development of the convention (Barthelmes 2015). The first mentioning was in 1996: “Conservation of peatlands”. At the Conference of the Parties in 1999 was the first acknowledgement of the importance of peatlands in climate change mitigation. In 2008 new resolutions confirmed that peatlands were the most important carbon store on land. The role of CH4 as a greenhouse gas was for the first time stated in a 2012 resolution. The Convention continues to have a strong focus on peatlands and their biodiversity and the link to climate change.
The United Nations Forum on Forest carries out a number of outreach activities to raise awareness of the multiple benefits of forests, and share best practices related to sustainable forest management. Work, is undertaken on the reporting on progress towards the implementation of the UN Strategic Plan for Forests 2030 and the UN Forest Instrument. At the heart of the Strategic Plan are six Global Forest Goals and 26 associated targets to be achieved by 2030. These include Goal 1: “Reverse the loss of forest cover worldwide through sustainable forest management, including protection, restoration, afforestation and reforestation, and increase efforts to prevent forest degradation and contribute to the global effort of addressing climate change.” And Goal 3: “Increase significantly the area of protected forests worldwide and other areas of sustainably managed forests, as well as the proportion of forest products from sustainably managed forests.”
Moreover, the Council of Europe’s Convention on the Conservation of European Wildlife and Natural Habitats (1979), or Bern Convention, was the first international treaty to protect both species and habitats and to bring countries together to decide how to act on nature conservation. Working on climate change mitigation through biodiversity solutions. The convention forms the basis for the Emerald Network in Norway and Iceland and it was the frontrunner of the two EU directives on nature i.e. the Birds and Habitats Directive.
2.3.4. The European Union
The EU has recently launched some rather ambitious plans directed by the President of the European Commission Ursula von der Leyen as indicated in one of her statements in 2020: “Making nature healthy again is key to our physical and mental wellbeing and is an ally in the fight against climate change and disease outbreaks. It is at the heart of our growth strategy, the European Green Deal, and is part of a European recovery that gives more back to the planet than it takes away."
The European Green Deal deals with climate change and environmental degradation, which are seen as an existential threat to Europe and the world and with a vision to develop a new growth strategy that will transform the Union into a modern, resource-efficient and competitive economy, where: 1) there are no net emissions of greenhouse gases by 2050, 2) economic growth is decoupled from resource use, 3) no person and no place is left behind and by a vision for turning climate and environmental challenges into opportunities.
The EU biodiversity Strategy for 2030 was launched in June 2020 by the EU Commission 2020 and its objectives endorsed by the Council of the EU in October. The strategy outlines biodiversity targets for the EU including an EU Nature Restoration Plan with a series of specific commitments and actions to restore degraded ecosystems across the EU by 2030, and manage them sustainably, addressing the key drivers of biodiversity loss. Of the 25% of the EU budget dedicated to climate action, a significant proportion is stated in the strategy to be invested in biodiversity and nature-based solutions as a mean to reduce GHG emissions. Furthermore, 30% of the land area should be protected and 10% strictly protected for biodiversity and with strict protection of the remaining primary and old-growth forests in the EU member states and with potentially legally binding nature restoration targets in 2021. Specifically, primary and old-growth forests are mentioned as the richest forest ecosystem that removes carbon from the atmosphere, while storing significant carbon stocks. Significant areas of other carbon-rich ecosystems, such as peatlands, grasslands, wetlands, mangroves and seagrass meadows should also be strictly protected, taking into account projected shifts in vegetation zones.
The two EU nature directives the Birds and Habitat Directives cover a large number of peatland and forest types categorized as habitat types or habitats for certain species and thereby protected by the directives. Habitat types under the Habitat Directive include raised bog, aapa and palsa mires, old broad-leaved deciduous forest and various types of temperate Beech forest to mention some examples included in the case studies. The Bird and Habitat directives are the cornerstone for the EUs nature conservation policy for the designation of Natura 2000 sites in Denmark, Finland and Sweden. Generally, the peatland and forest habitats in the Nordic EU countries are in an unfavorable conservation status according to the EU reporting in 2019.
Moreover, the Water Framework Directive plays an important role in the protection of inland surface and ground waters and to restore good ecological status including a high-water quality. In the Nordic countries there is a general need to reduce the N and P pollution in water courses and lakes in order to meet the requirements of the directive. The directive explicit refers to the restoration of wetlands, which includes rewetting of peatlands. The EU Agricultural Policy (CAP) is important for the land use in EU including the Nordic EU countries. The CAP includes the EUs largest subsidy scheme involving hundreds of billion euros for agricultural support under pillar I and II. The second Pillar focus on environmental sustainability and climate and will be increasingly relevant for providing incentives for restoration measures in peatlands and forests.
The EU 2030 climate policy framework includes the LULUCF (Land Use, Land Use Change and Forest) Regulation (Romppanen, 2020), which concerns carbon pools in above-ground biomass, below-ground biomass, litter, dead wood, soil organic carbon, and harvested wood products in the land accounting categories of afforested land and managed forest land. In contrast to previous EU law, where emissions from use of biomass in energy production were not accounted, the LULUCF Regulation does include biomass for energy production: https://ec.europa.eu/clima/policies/forests/lulucf_en. EU Forestry Policy.
3. Synergy between biodiversity and climate regulation
The assessed synergy from different management options and their effects on biodiversity and climate, is provided in Tab. 3.1 and 3.2.
The two tables summarize our assessments based on general scientific knowledge as well as input from the eight case studies (presented in chapter 6). Here we elaborate on the rationale behind these assessments and discuss synergies in this context. These considerations do also form a basis for the assessments of synergy in the eight case studies although cases differ in the level of details and type of information.
The overall assessment of synergy between biodiversity conservation/restoration and mitigation of climate change follows a simple colour codex of green (yes), orange (depends) and red (no). The green colour indicates synergy. Red colour indicates no synergy because either biodiversity or climate or both do not benefit from the management and the orange colour indicate that synergy is context specific and depends on a specific situation or the assumptions set for the assessment and/or calculations.
Guiding towards the assessment of synergy is the effect on biodiversity and climate assessed separately and following a colour codex. Here the greenish colour indicate strong positive effect on biodiversity or climate from a given action and the reddish colour a strong negative effect. A positive effect is being graduated as strong (green) or weak positive (light green) as well as strong (red) or weak (light red) negative. Orange depends on the chosen baseline or the kind of climate effect.
The effect on the climate is split in 1) the effect on the preservation of the carbon stock (or tree biomass) and 2) the effect on carbon uptake and sequestration by the plants (see Tab. 3.1 and 3.2 for the results).
3.1. Peatlands
Synergy between biodiversity conservation and climate change mitigation is identified when drained peatlands are restored by rewetting and reestablishment of natural hydrological conditions. This can be achieved by blocking ditches, cutting down trees or building dams (Joosten in press., Dinesen & Hahn 2019) to secure water in the peatland and avoid drainage to the surrounding land, which is often drained agricultural land or managed forests. Rewetting of the organic soil stops the emission of CO2 and restores the conditions, which are the foundation for unique biological communities in mires. Rewetting all types of peatlands creates synergy. This ranges from living peatlands, also called mires, to cultivated organic soils, which have lost their original biological communities. Living peatlands slowly build up carbon stocks, which entails a long-term climate benefit, although their greenhouse gas balance is often rather close to zero and they may act as both sources or sinks over time.
Restoration with no active interventions may yield positive results in certain cases for example in old poorly drained boreal peatlands where forestry is abandoned. Synergy actually may develop without human intervention because the old ditches created for forestry slowly fills in resulting in a gradually rise in water table, which is good for the climate because it prevents the aerobic decomposition of organic material and resulting emissions of CO2. At the same time biodiversity will benefit from increasingly wet conditions and the abandoned wood production, which mean that fertilizer is not added and discontinued logging mean that a burst of these nutrients to the water is also prevented. The synergy in nutrient poor peatlands will be largest from no interventions while for example nutrient rich fens will have larger emissions and synergy will be dependent on restoring a high-water table as soon as possible to stop CO2 emissions. Hence, a flexible approach at landscape level will provide the largest opportunities for creating synergy because it depends on the specific setting of the individual peatland.
On the other hand, no intervention in abandoned agricultural peatlands or existing agriculture on organic soil for that matter extend the negative effect on climate due to the emission of CO2. At the same time biodiversity is maintained and not being restored due to a lack of water and a heavily degraded state. The introduction of paludiculture i.e. crops on wet soil or in water is the only solution to change this negative impact. Paludiculture will benefit climate per definition (see Wichtmann et al. 2016), however, the benefit for biodiversity may dependent.
Agriculture and forestry on peat soil has negative effects on biodiversity compared to the more pristine situation. Generally, both activities also negatively affect climate, although in some cases the effect of forestry may be debated (see below). Peat excavation is usually dependent on drainage and is directly devastating for biodiversity and climate because of drainage and even more so when the peat is used for energy. In that regard using peatlands as a source of energy is nothing better than using fossil fuels, however, the biological communities are lost as well when energy comes from peat.
Tab. 3.1. Overview of the expected effects of different peatland management options on biodiversity and climate, and the possible synergies. The effect on climate (column 5) is the combination of the carbon stock and uptake and emission of GHGs (column 3 and 4). The specific effects are briefly described in each cell. Colour legend are given below. See text for further explanation.
| Legend, effects: |
| Strong positive effect |
| Weak positive effect |
| Depends on chosen baseline or the kind of climate effect |
| Weak negative effect |
| Strong negative effect |
| Legend, synergy: |
| Yes |
| Depends |
| No |
| Management option | Effect on | Synergy between biodiversity and climate | |||
| Biodiversity | Carbon/CO2 (+ methane, CH4) | Climate | |||
| Stock (In ecosystem) | Uptake and emission (including sequestration) | ||||
| Conservation of intact mire system (pristine peatland) (Case studies #1,# 2,#3, #4) | High biodiversity is conserved | The large carbon stock is preserved and slowly increases | Uptake low, some years may even be negative but exceeds GHG emission from the mire in the long-term | The large carbon stock is preserved. Accumulation of carbon in the long-term | Important for biodiversity and effectively stored carbon |
| Restoration of a mire ecosystem (active peatland with natural vegetation) by rewetting and e.g. removal of exotic trees. (Case studies #1,#2, #4) | Biodiversity is conserved and increases | The (remaining) large carbon stock is preserved and slowly increases | Significant emission of CO2 decreases or stops. CH4 increases. Uptake low but continue in the long term | The remaining large carbon stock is preserved. GHG emissions reduced, at least in the long-term. Accumulation of carbon may restart. | Important for biodiversity and effectively stored carbon |
| Restoration of degraded peatland without natural peat communities by rewetting and e.g. facilitation of spaghnum growth (Case studies #1, #4) | Biodiversity increases, but only slowly, especially for demanding species | The (remaining) carbon stock is preserved | Significant emission of CO2 decreases or stops. CH4 increases. Uptake low or zero but may build up in the long-term | The remaining carbon stock preserved. GHG emission reduced at least in the long-term. | Yes. But effects may be slow for biodiversity and for climate it depends on size of carbon stock left. |
| No restoration intervention on poorly drained boreal peatlands with semi-natural vegetation on abandoned forestry land (Case study #4) | May benefit biodiversity, but slow recovery and speed of development of biological communities may depend on e.g. water quality | The remaining carbon stock may be preserved due to natural rise in water level but nutrient rich peatlands may be emitters | Some sequestration from planted trees and, in the long term, from recovered peat due to increase in water level | The remaining carbon stock preserved depending on nutrient status. GHG emissions reduced due to increase in water level. | Slow recovery of biodiversity compared to restoration scenario and preserved carbon stock dependent on specific setting |
| Mainstream forest management on peatsoil including drainage and use of fertilizer (Case studies #2, #4) | Biodiversity reduced and changed | Stock reduced from natural level | Uptake increased by forestry, but may be be counterbalanced by emissions from peat decomposition | Stock decreases, but wood production may entail climate benefits | No |
| Conventional agriculture on organic soil (peatsoil) (Case study #1) | Biodiversity markedly reduced and changed | Stock continues to deterioate for a long time unless changed to paludiculture | Emission large | The carbon stock decreases and emission continues unless changed to paludiculture | No |
| Peat excavation (Case studies #1, #4) | Biodiversity destroyed | Stock destroyed | Emission large | Stock decreases and with large emissions | No |
3.2. Forests
Most importantly, we identify synergies between biodiversity conservation and climate change mitigation with both conservation of natural old growth forests and restoration of (more) natural forest ecosystems in deforested areas or managed forests. These synergies are discussed below and further documented in the case studies. For biodiversity, the basic assumption is that natural undisturbed old growth forests (i.e. unmanaged forest) with a broad array of natural habitats host the highest and most important biodiversity including the highest richness of rare species. For biodiversity at species level, this is well documented in the field (e.g. Berg et al. 1994, Christensen & Emborg 1996, Paillet et al. 2010, Müller & Bütler 2010, Rudolphi & Gustafsson 2011, Lelli et al. 2019). However, it also lies in the fact that natural ecosystems per se are important elements of biodiversity according to the general definitions by CBD and IPBES as outlined above. It is also well-established that logging, and forestry and commercial forest management in broader terms, entails negative impacts on biodiversity, although the magnitude depends on the specific management practices (cf. the references above). In accordance with the above, both preservation of old growth forests and schemes allowing managed forest to develop towards natural old growth forests is widely accepted as important and effective biodiversity conservation measures (Crouzeilles et al. 2016, Lindenmayer et al. 2006).
The climate mitigation effects are derived from two components: The ecosystem carbon stock (in biomass and soil) and the net uptake of CO2 from the atmosphere and storage of carbon (carbon sequestration). It is well established that the carbon stocks are generally larger in natural old growth forest than in a managed production forest where trees are on average younger and smaller due to logging (Harmon et al. 1990, Ciais et al. 2008, Mäkipää et al. 2011). Thus, there is a clear climate benefit from protecting old growth forests because large natural carbon stocks are effectively preserved and CO2 is kept out of the atmosphere. This also means that forest reserves in previously logged forests typically entails carbon sequestration when wood is no longer harvested and (more) natural carbon stocks build up (e.g. Allen et al. 2016, Braun et al. 2016). On the other hand, as mentioned before, the harvest of wood products in managed forests may contribute to climate mitigation by substitution of fossil fuels and energy-intensive materials like concrete and steel, but also through the storage of carbon in buildings and long-lived wood products (Sathre et al. 2010, Leskinen et al. 2018).
It is currently highly debated as also mentioned before, whether forest conservation or management for wood production entails the largest climate benefits (e.g. Searchinger et al. 2018, Taeroe et al. 2017, Nabuurs et al. 2017). The answer to this strongly depends on the time perspective and numerous other assumptions including the expected development within forest management and in several other sectors. Examples are the future efficiency of timber production, the use of wood for construction and developments within emission-free energy sources such as wind and solar energy. This uncertainty is indicated with orange marking of the relevant cell in Tab. 3.2. Concluding on this issue is beyond the scope of this study. We limit our assessment to the facts that there are clear climate benefits associated with restoring (more) natural forest ecosystems in previously managed forests.
We also identify synergies associated with afforestation, i.e. planting or natural regeneration of forest in previously non-forested or deforested areas. Thus, afforestation will almost inevitably entail carbon sequestration wherever it is implemented (increase of carbon stocks), and climate benefits may also arise from harvested wood products in the long term. Afforestation on farmland or degraded land will most often also benefit biodiversity in the long term, but the magnitude and nature of these effects will depend on the geographical context and very much on the future management. For example, natural hydrology should always be restored in drained areas. On the other hand, afforestation on natural open habitats like grasslands, heathlands and bogs entails no synergy. Although it might benefit the climate (if no drainage is applied), the associated changes of the natural biodiversity must be regarded as a negative impact.
Hence, the orange cells in Tab. 3.2 indicate that the assessment of effects or synergies and trade-offs depends on the context, the assumptions or the baseline, i.e. the state of the forest with which the management scheme in question is compared. An example of the latter is implementation of conservations actions in managed forest, which might entail positive effects on biodiversity, while forestry as such still negatively affects the biodiversity. Another example is the assumption concerning carbon sequestration: Are old growth forest carbon sinks in general, and for how long does the ecosystem carbon sequestration continue in a forest after logging is stopped? This is also debated and subject to further research. Yet another example is the above question, whether managed or unmanaged forest best mitigate climate change. Further research and implementation of demonstration projects are needed.
Tab. 3.2. Overview of the expected effects of different forest management options on biodiversity and climate, and the possible synergies. The effect on climate (column 5) is the combination of the carbon/CO2 stock and uptake (column 3 and 4). The specific effects are briefly described in each cell. Colour legend are given below. See text for further explanation
| Legend, effects: |
| Strong positive effect |
| Weak positive effect |
| Depends on chosen baseline or the kind of climate effect |
| Weak negative effect |
| Strong negative effect |
| Legend, synergy: |
| Yes |
| Depends |
| No |
| Management option | Effect on | Synergy between biodiversity and climate | |||
| Biodiversity | Carbon/CO2 | Climate | |||
| Stock (In ecosystem) | Uptake (sequestration and harvest) | ||||
| Conservation of old-growth forest (Case studies #6, #7, #8) | High biodiversity is conserved | The large stock is preserved | Uptake low or zero | Carbon stock is preserved. But no or only little gain | Yes. But most important for biodiversity |
| Restoration forest ecosystem (From managed forest to old-growth in the long term) (Case study #7) | Biodiversity is conserved and increases | Stock increases (sequestration) | Uptake decreases over time, possibly towards zero | Ecosystem stock increases (and is larger than without forest), but climate benefits from wood production is lost | Yes. But for climate, only in terms of ecosystem carbon stock |
| Restoration through reforestation (Natural regrowth or planting. Old growth in the very long term (Case studes # 5, # 6) | Biodiversity increases, but only slowly, especially for demanding species | Stock increases (sequestration) | Uptake increases, but decreases again in the long term | Stock increases | Yes. But effects are slow, especially for biodiversity |
| Afforestation for wood production (on farmland or degraded land) (Case study #6) | Biodiversitety increases, but mainly common species | Stock increases (sequestration), but not to natural level | Uptake increases, but decreases again in the very long term | Both stock and upptake increases | Yes, but effect largest on climate |
| Biodiversity conservation in managed forest (Case study #8) | Benefits biodiversity, but still reduced by forestry | Stock slightly increase, but still below natural level | Uptake slightly reduced, but still above natural forest, and is maintained in the long term | Small increase of stock (but still below natural level). Climate benefits from wood production is retained but slightly reduced | Depends on the baseline (starting point) and several other considerations |
| "Mainstream" forest management | Biodiversity reduced from the natural level | Stock reduced from natural level | Uptake increases and is maintained in the long term | Stock decreases, but wood production entails climate benefits | No |
| Management for increased biomass production | Biodiversity reduced (further) from the natural | Stock reduced (slightly more) from natural level | Uptake increases (further) and is maintained in the long term | Stock decreases, but wood production entails (increased?) climate benefits | No |
4. Nordic Policy options
The following ten policy options are provided based on the examination of the eight cases and the literature behind and the scientific literature in general as well as an examination of the current policy agreements. The headlines in bold are presented in the executive summary but elaborated in the following. Numbers do not reflect prioritization.
1. Restoring nature, as exemplified in this report, provides an excellent option for Nordic countries to align with and take lead in meeting international biodiversity and climate policy targets through nature-based solutions.
Preserving natural ecosystems and restoring degraded ones are important to combat the biodiversity and climate change crises. Not only are well-functioning natural ecosystem essential to biodiversity, they also store carbon and emit less greenhouse gases than disturbed ecosystems. Land sparing to ensure protection and restoration of peatlands and old-growth forests are two of the main tools to reach the targets of the Convention on Biological Diversity, the Convention on Climate Change and the EU Biodiversity Strategy. These include nature protection on 30% of the land by 2030 and phasing out net greenhouse gas emissions in all sectors by 2050. The Nordic countries are in a good position to align with and provide leadership with regard to these policy targets because of generally high standards of education, democracy and resources. Documentation of progress and clearly defined deadlines will be crucial to reach targets.
2. A stop for new drainage activities in mires is essential to preserve natural carbon stocks and conserve biodiversity.
Mires effectively store large amounts of organic carbon in the soil. Therefore, draining causes large emissions of CO2 as these stores are exposed to oxygen. Draining also degrade or destroy important natural habitats for a multitude of species. Policies to limit future draining must include options for phasing out peat products including Sphagnum for horticulture and peat for energy production and take into account that agriculture and forestry as well as infrastructure development cannot expand further in such areas.
3. Nordic countries can prevent emissions of carbon dioxide and initiate long-term biodiversity restoration by rewetting drained mires and peatlands including areas currently used for farming, forestry or peat excavation.
Large areas of Nordic peat lands are used for farming, forestry or peat extraction for fuel or horticulture. Rewetting such areas as an initial step of restoration is an efficient way to reduce emissions of carbon dioxide, because it will halt the decomposition of the peat soils and reduce emissions of carbon dioxide. Although rewetting may cause emission of methane, which is another greenhouse gas, large overall climate benefits will be achieved. Whereas, biodiversity will increase in restored mires; biodiversity will recover more slowly in degraded “non-active” peatlands.
4. Strict protection of existing old growth forests by the exclusion of forestry will safeguard important biodiversity and contribute to climate change mitigation through the preservation of natural carbon stocks.
Almost all natural forest ecosystems in the Nordic region has been subject to degradation, mainly through logging and widespread drainage of wetlands and moist soils and subsequent transformation into farmland or managed production forest. This in turn has led to large-scale habitat destruction, loss of biodiversity and emission of CO2 due to decomposition of natural carbon stocks. Putting an end to this development in the Nordic countries is essential and will be a strong signal to the global community.
5. Restoration of forest ecosystems allowing them to develop towards natural old growth forests is essential to biodiversity conservation and contributes to climate change mitigation and ecosystem resilience.
Due to their currently very limited distribution, increasing the area of unmanaged forest is a powerful tool to conserve biodiversity, while climate benefits are achieved through carbon sequestration in trees and soil. The most efficient approach is to establish non-intervention reserves in presently managed forest, because the habitats associated with old growth structures will develop fastest there. Only in such areas, at scale of landscapes, is it possible to restore the full array of natural habitats for different organisms. Initial reforestation on historically deforested lands is the next option, especially on existing or abandoned farmland, which is usually of low value to biodiversity. Afforestation or reforestation can be done by either natural regeneration or planting of native tree species, but areas for planting should be carefully assessed and natural habitats should be avoided. Rewetting of drained wetlands in the forest is essential for biodiversity and does contribute to climate change mitigation.
6. Conservation actions in managed forests can be a supplementary option, which is significantly less effective for preserving biodiversity, but with the potential to obtain or retain the possible climate benefits and economic return from forestry.
This option is particularly relevant if the prospect for income can facilitate afforestation in heavily degraded areas that would not otherwise be reforested and the potential biodiversity and climate benefits thus not be achieved. Actions that benefit biodiversity in existing managed forests can be a supplementary, but less effective, option to obtain synergy with climate mitigation. However, this cannot replace protected forest reserves with no logging, which is an indispensable part of an overall conservation strategy, and the most cost-effective approach. Only in such areas, at the scale of landscapes, is it possible to restore a full array of natural habitats for different organisms.
7. Improved documentation of the greenhouse gas dynamics and biodiversity of intact and restored ecosystems preferably at the same location is essential to develop informed and efficient nature-based solutions and strategies.
Today, the assessment of intervention scenarios in drained wetlands often rely on IPCC standard values, although these are very uncertain and only intended for indicative assessments at the national level. Similarly, the assessment of future carbon stocks in forest reserves often rely on very few measurements in intact old growth forests. Moreover, coherent documentation of biodiversity and climate parameters at the same locations are, unfortunately very scarce; both in natural ecosystems and concerning the effects of conservation and restoration initiatives. Thus, the more widespread measurements of greenhouse gas fluxes and carbon stocks as well as targeted biodiversity studies will substantially increase our understanding of the climate and biodiversity in restoration of hydrology and establishing of no-intervention forest reserves.
8. Planning at large spatial scales will increase the efficiency and facilitate both local and overall synergies between biodiversity conservation and climate change mitigation.
Prioritization at landscape, regional or national scales of different management interventions in different forest and wetlands is an important planning tool. The assessment of existing values and potentials of a large number of areas can identify where to focus mainly on either biodiversity or climate, or where one can exploit real local synergies. In this way, the greatest possible overall synergy is obtained. Explicit consideration of the lost economic income of setting different areas aside (opportunity costs) will further increase the feasibility and cost efficiency of the plans. In addition, setting aside large coherent areas for biodiversity conservation (natural landscapes) provides the opportunity to conserve the full array of habitats and biological communities that are present in intact ecosystems.
9. Enhanced national mechanisms to provide advice and communicate results of scientific research on biodiversity and climate change will improve decision-making and public debate.
There seems to be a need to strengthen the policy-science interface at national level in the Nordic countries. This could include establishing independent scientific advisory boards and tapping effectively into international knowledge platforms and results from pilot projects and documentation of practical implementation. The establishment of e.g. national support units linked to knowledge-based processes of IPBES and IPCC provides an opportunity to strengthen science-policy interface and communication to the public. Enhanced dialogue and cooperation between research, authority and management units across biodiversity and climate will promote synergetic solutions.
10. Ambitious cross-sectorial and cross-disciplinary policies can facilitate the wider use of cost-efficient nature-based solutions to meet the biodiversity and climate challenges.
Focusing on the technical and scientific aspects, this report presents examples of promising Nordic nature conservation and restoration initiatives that benefit both biodiversity and climate. However, the implementation of such nature-based solutions at larger scales require policies that address a multitude of wider aspects including the following:
- Integration across sectors including e.g. agriculture, forestry, tourism, trade and industry
- Consideration of both climate, nature, water and socioeconomic aspects
- Participation and involvement of stakeholders at the local level
- Support from the governing bodies at local, regional, national levels
- Incentives to stakeholders at all levels to support nature-based solutions
- Public awareness of the need for action and the wider positive perspectives of the solutions.
- Review and revision of legislation in the light of the new biodiversity and climate policies to eliminate regulations and perverse incentives that prevent or limit the necessary measures and changes for synergy.
5. Introduction to case studies
Eight case studies are presented in this report from five Nordic countries. Information on the process and criteria for choosing the cases is found in chapter 2.2. It is important to stress that no field data collection have been undertaken by our project hence we have solely relied on existing information.
Peatlands
Four peatland cases were selected from four different Nordic countries: Denmark, Finland, Norway and Sweden. Three of these are EU LIFE funded projects related to restoration or land use options and the fourth from Norway include studies and long-term monitoring of palsa mires influenced by permafrost. The cases differ from focus on a single locality in Denmark to a range of sites in Sweden to regional approach in Finland and a full habitat type in the case of Norway. Measurements of greenhouse gas fluxes have been conducted in one of these projects only while biodiversity surveys have been undertaken in relation to all cases, however, in very different ways.
Different peatland types in different climate zones are covered by these four cases. First of all, the cases include raised bogs, fens as well as aapa and palsa mires in the temperate, boreal and sub-arctic zones respectively. Moreover, active mire restoration activities are conducted and described in relation to two of the cases in Denmark and Sweden while peatland restoration is also taking place in Finland, however, this case is based on an outline of future management options in a large region of boreal Finland. The fourth case, from Norway, is special in the sense that limited management options exists at site level. The general challenge here is the rapid changing climate and its effects on (sub)arctic peatlands, which again can result in cascading effects and feedback loops, which are unpredictable based on current knowledge.
Forest
Four forest cases were selected: Denmark, Iceland, Sweden and Norway. These cases comprise also different vegetation zones from temperate forests in Denmark and southern coastal Norway to boreal forests in Sweden and subarctic and boreal birch woodlands on Iceland. Biodiversity studies of varying scope were available in all cases. Field based estimates of biomass carbon stocks were also available in all cases, while estimates of soil carbon in some cases relied on model estimates. Past and/or future carbon sequestration estimates were obtained based in different approaches. Direct measurements of greenhouse gas fluxes were not available.
The two case studies from Iceland and Norway, respectively, present the potentials of restoring native deciduous forest ecosystems that have been converted in the Norwegian case for agriculture. Focus is on areas capable of natural or assisted regeneration in abandoned landscapes with minor human management. A third case from Denmark presents an agreement to restore old growth forests in a long-term perspective. Focus is on the databased prioritization of future conservation area where commercial forestry will be abandoned after initial restoration interventions. The fourth case from Sweden presents research in ancient boreal forests of different age classes spanning several thousand years. Focus is on the accumulation of organic carbon in tree biomass and soil, and the development of biological communities, in long term undisturbed ecosystems (reference locality).
The eight cases presented in chapter 6 are:
6.1. Restoration of one of the largest raised bogs in lowland northwest Europe, Denmark
6.2. Restoration of natural landscape hydrology - mire restoration, Sweden
6.3. Palsa mires – a threatened sub-arctic habitat, Norway
6.4. Future land use options in peatlands with abandoned forestry, Finland
6.5. Natural regeneration of temperate deciduous forests, Norway
6.6. Restoration of an almost extinct forest ecosystem, Iceland
6.7. Doubling the area of forest set aside biodiversity for conservation, Denmark
6.8. Development of biodiversity and carbon storage in ancient boreal forests, Sweden
A summary of the synergy between biodiversity and climate change mitigation is found in Tab. 3.1. and 3.2. with an indication of where the information from each of the cases fits into this assessment.
6. Case studies
6.1. Restoration of one of the largest raised bogs in lowland northwest Europe, Denmark
Introduction
Focus in this case is on the large-scale restoration efforts going into Lille Vildmose i.e. one single locality with a primary aim to improve and reestablish biodiversity most notably raised bog habitat. At the same time Lille Vildmose has been a model and demonstration site for climate change mitigation at the international level through the Ramsar Convention.
Lille Vildmose is a large peatland complex that covers one of the largest areas with active raised bog in lowland northwest Europe. Today approximately 21 km2 of raised bog habitat remains – more than one-third of its original size. The bog was until about 2,500 years ago part of a strait connected to the sea. The landscape elevated due to post-glacial uplifting and eventually the strait was blocked and a brackish lagoon developed covered by nutrient-poor reed swamp. The reed swamp and subsequent development of forest bog were followed by treeless bog of Sphagnum mosses. The Sphagnum eventually lost contact with the groundwater creating a raised bog persistent to present.
Until human activities started in c. 1760 Lille Vildmose comprised a blend of bogs, forests, lakes, and meadows. The largest part was bog. The area was then a 55 km² large raised bog surrounded by a narrow wet zone “lag zone” with a few small streams running from the bog area. Originally, several separate lakes were located in the bog covering more than 4 km² surrounded by peat habitat and with a natural outlet to the sea.
Between 1760 and 1769 the lakes were drained and the lake bottoms started to be used for agriculture. Handmade canals were excavated over the years including establishment of a 7 m deep and 2 km long canal draining the lake water straight to the sea (see also Hansen 2011). Two of the lakes were much later restored in 1927 and 1973 and a third in 2019. In contrast to the acid bog, the freshwater lakes had a neutral pH as they were fed by groundwater springs connected to calcium-rich soil.
The bog was still largely intact until 1937-39, when the Danish government acquired 23 km2 in the central part with the objective of creating farmland for small-scale farmers. Digging 200 km of ditches improving the drainage and the cultivation begun (Hansen 2011). Other activities included extraction of marling, a friable earthy deposit consisting of clay and calcium carbonate used as a fertilizer for soils deficient in lime and peat-extraction for energy including for the local cement-industry.
After World War II the cultivated land was found to be rather unattractive. Of 80 planned areas for small-scale farmers, only a minority was sold. Much of the area turned into grassland and was used for summer grazing by livestock. The government started to lease land for peat-extraction. Initially this was mainly for fuel, but later it developed into a highly industrialized extraction of Sphagnum for private and market gardening.
Both the northern and southern area were fenced in 1906 and 1933–34 including active raised bog areas as well as two forests and they used to be hunting grounds targeting with semi-wild populations of Wild boar (Sus scrofa) and Red deer (Cervus elaphus).
The private nature foundation Aage V. Jensen Nature Foundation has since 1988 bought land in the Lille Vildmose area and in 2001–2004 the large part of remaining bog habitat as well as land in between were purchased by the foundation (Hansen 2011). This has resulted in a dramatic change in land use in the last 2–3 decades with the primary aim now being to secure and restore natural values and to allow visitors to enjoy and learn from and gain insight into these values and functions.
Case
A total of approximately 50 million Danish kroner has been invested in restoration activities from EU LIFE funds and the private foundation and landowner Aage V. Jensen Nature Foundation, the Danish Nature Agency and the municipality of Aalborg from 2011–2020. Moreover, the expenses to compensate landowners through a conservation act was estimated to at least another 50 million Danish kroner (R. Poulsen pers. comm.). The overall objective of the restoration project has been first of all to secure existing areas of raised bog, secondly to rewet former degraded and excavated areas, and thirdly to reconnect various functional hydrological units in the area. One key target area for restoration has been the large and heavily degraded central part of the former bog situated between existing bog habitats in south and north and north-west (Fig. 6.1.1). Some of the major activities included:
- Reestablishment of a 1.3 km2 large lake
- The release of Red Deer in the central area
- Restoring wet conditions in large parts of the central area
- Prevent further drainage of the three largest existing raised bog fragments in north and south by building more than 3 km of dams along the bog margins.
- Mitigate the accelerated growth of shrubs and trees in two semi-large raised bog fragments
- Persecution of invasive Racoon dog (Nyctereutes procyonoides) and American mink (Neovison vison)
This large-scale investment had first-and-foremost the aim to prepare the area for hydrological restoring of the raised bog habitat towards its former distribution. In terms of costs of activities, it was a rather low cost action to restore a high water table (immediately benefitting climate) and much more expensive to remove shrub and treed and establish fences for controlling the movement of large grassers such as Red deer and Moose (Alces alces) (se later). Overall, the most expensive part has been compensation or buying out landowners.

Fig. 6.1.1. Aerial photo of Lille Vildmose (2012) with some of the major bog restoration areas delineated by red lines and the delineation of the Ramsar site (corresponding to an EU Bird Area) in yellow. Solid dark brown areas within the Ramsar boundary are active raised bog habitat (the largest situated in south). An important part of the restoration activities was also to keep water in the existing bog by building dams towards adjacent agricultural land. Provided by Peter Hahn, Danish Nature Agency.
Land tenure and protection status
The Aage V. Jensen Nature Foundation is a private nature conservation foundation and the biggest landowner. Thus, the far majority (5,881 ha) of the land is owned by this private foundation, with an aim to conserve and restore biodiversity including the re-establishment of areas of former raised bog. The peat extracting company Pindstrup Mosebrug is the owner of 474 ha and the second largest owner. A number of private owners and Aalborg Municipality (30 ha) own the remaining peatland.
An EU LIFE project was implemented 2011–2020 with the primary aim to restore raised bog habitat i.e. an unambiguous biodiversity focus. The peatland area and adjacent forest areas are covered by the largest nature conservation order in Denmark comprising 7,513 ha. Moreover, wetland habitats are generally protected under the general Danish Nature Protection Act (article 3) protecting fens, bogs, meadows and heath larger than 2500 m² and lakes above 100 m².
Lille Vildmose has Natura 2000 status and the area is covered by both the EU Birds and the Habitats Directives. It was given Ramsar status in 2013 with the aim to spearhead the restoration of peatlands worldwide for climate change mitigation by using key areas as awareness raising and demonstration sites (Barthemeles et al. 2015, Ramsar 2015, Ramsar 2018). This designation promoted the use of climate regulation as an additional argument for peatland designation within the convention.
Ramsar specifically promoted the importance of peatlands to mitigate climate change by several resolutions, which were led by the Nordic countries in collaboration and Lille Vildmose was used as a model to promote this concept at international meetings of both the Ramsar and the Climate Conventions (see e.g. Hahn 2015).
Current land use and management
Peat extraction ceased within the Natura 2000 area around 2011 and only excavation in a small area outside the Natura 2000 area continued. Today the central part, north of the active raised bog, is a mixture of open permanently rewetted peat-mines and extensive grassland. Isolated to the west, north and northwest are three remnants of raised bogs – all three partly degraded and recently rewetted (Fig. 6.1.1) as part of earlier restoration initiatives.
All-together the most important natural habitats are active raised bog (approx. 21 km2), bog woodland (approx. 4 km2) and old natural forest of high biodiversity value (approx. 10 km2). Peat extraction has taken place in large parts of the area thereby reducing the area of active raised bog from originally approximately 55 km2 (see Fig. 6.1.1). The surrounding land is cultivated and with scattered settlements and villages.
Drainage of farmland including dredging of an important local stream (Haslevgaarde Å) adjacent to the largest remaining raised bog area in south progressively cause this to dry out. This in turn has allowed colonization of Birch (Betula sp.) and to a minor degree conifers which have increased evaporation.
Restoration activities have included blocking of numerous ditches and canals, establishment of dikes with waterproof membranes, restoring of a lake and elimination of especially Birch and to a less degree Alder (Alnus glutinosa) on 2 km2 and on more than 7 km2 Birch and to a less degree Willow (Salix sp.). Former peat extraction areas have included work to restore damming of drainage-systems of 7.7 km2 in order to retain water and/or reduce outflow and resulting in significant areas being flooded. Moreover, re-growth of Sphagnum moss has been actively facilitated in small experimental sites (10 ha) and it has started to naturally colonize many parts of the area. These actions have resulted in a significant enlargement of areas in process towards self-regulating natural habitats such as natural dystrophic lakes and ponds, transition mires, and quaking bogs as well as bog woodland – and in the long-term towards active raised bog habitat.
In parallel to these physical restoration activities a large-scale on-going grazing and rewilding project is under implementation. A fence around the central area covering 21 km2 allows trials with free ranging Red deer and Moose – the latter introduced as recently as 2015. Thereby providing the opportunity to combine three separate fences in the area potentially allowing movement of large herbivores between sections in a multispecies grazing regime. The fenced area in the northern Høstermark is 4.7 km2 and in south Tofte mose 39.7 km2. The purpose is partly to introduce keystone species to promote natural ecosystem development by keeping the former peat extracted areas and the margins of the raised bog areas open by limiting the overgrowth.
It has been a high priority when implementing activities to ensure a high level of information and a number of information boards, boardwalks, and wildlife hides and towers have been established and meetings to inform landowners and stakeholders held. A visitor center is located within the area.
Stakeholders
Apart from the three project implementers and private landowners a large number of visitors is touring Lille Vildmose (see below).
Some landowners have been strongly against the restoration activities. This has delayed restoration and is hampering the effects of a general rise in e.g. water level because the raised bog habitat was still drained at its edges towards the agricultural land. Some of these landowners and “neighbours” remain skeptical to the project and they have delayed restoration activities considerably. However, not all landowners have been against the restoration project and field excursions have been arranged a couple of times each year to present the current activities for landowners and other stakeholders.
The area is visited by a large and increasing number of nature tourists and civilized explorers now numbering more than 150,000 persons a year (in 2019), with an interest in nature and wildlife. Pupils from schools from nearby towns or the larger city of Aalborg some 30 km away are educated using the area and the visitor and education center. Especially the introduction of Moose has attracted many visitors in recent years.
Climate
Emissions
Land use types in Lille Vildmose are documented in detail. Thus, it is possible to calculate greenhouse gas emissions using accurate area estimates for the various land use categories following the IPCC guidance (IPCC Wetlands Supplement 2014). These are theoretical emission values from the IPCC as there are no area specific flux measurements available. Land use categories are listed in Tab. 6.1.1 and comprise in the calculations: 2,022 ha of active raised bog, 252 ha of degraded raised bogs still capable of natural regeneration, 1,246 ha of degraded peatland under restoration, 400 ha of bog woodland and 1,000 ha of old natural forest on mineral soil.
Calculation of emissions using the default values for tier 1 (IPCC Wetlands Supplement 2014), arrived at net GHG emissions of 17,780 CO2-eq. per year before the major restoration activities started in 2011 (Tab. 6.1.1) reduced to 7,294 CO2-eq. per year after restoration. Thus, a reduction of approximately 10,500 tons CO2-eq per year, due to the rewetting. Although this presents a rough calculation (see also chapter 2.2.) with a degree of uncertainty this is the best evidence we have at present.
Lille Vildmose will continue to be a net greenhouse gas emitting ecosystem, but at a much reduced level. CH4 emission is happening from intact and rewetted peatlands because microbial respiration changes from aerobic to anaerobic resulting in a shift from CO2 to CH4 emission. CH4 emission is also a greenhouse gas resulting from anaerobic respiration and is taken into account in these calculations (see Barthelmes et al. 2015 and chapter 2.2. for more information on this issue).

Fig. 6.1.2. Rewetting in former drained and excavated peat area. The restoration of a more natural hydrology has resulted in a reduction of GHG emissions at the scale of approximately 10,500 ton CO2 –e yr1 due to the cease of CO2 emissions from the organic soil (Barthelmes et al. 2015). The results from biodiversity monitoring programs strongly indicate that biodiversity is also recovering (Naturstyrelsen 2013). Photo: Jens Vinge.
Carbon storage and sequestration
The estimated soil organic carbon in Lille Vildmose is approximately 7.4 million ton or approximately 10% of the Danish peat carbon volume (Joosten 2009). In the mire in the southern part (Tofte Mose) it has been calculated that 4.5 million ton C (4.5 m thick peat layer x 20 km2 of land x 0.1 dry peat weight x 0.5 carbon content) are stored and the storage of this organic soil is totally dependent on continued water logged conditions.
| Before rewetting | Area (ha) | Emission factor (ton CO2 -e ha1 yr1 | Total emission Ton CO2 –e yr1 |
| Active raised bog | 2,022 | 0 | 0 |
| Degraded raised bog | 252 | 10 | 2,520 |
| Degraded peatland | 1,246 | 10 | 12,460 |
| Bog woodland | 400 | 7 | 2,800 |
| Total | 17,780 | ||
| After rewetting | Area (ha) | Emission factor (ton CO2-e ha1 yr1 | Total Ton CO2 –e yr1 |
| Active raised bog | 2,022 | 0 | 0 |
| Degraded raised bog | 252 | 3 | 756 |
| Degraded peatland | 1,246 | 3 | 3,738 |
| Bog woodland | 400 | 7 | 2,800 |
| Total | 7,294 |
Tab. 6.1.1. Green House Gas Emissions before and after reestablishing of hydrology. Based upon standard emission factors for rewetted peat soils from the IPCC Wetlands Supplement (IPCC 2014). Source: Barthelmes et al. (2015).
Ramsar Information
Looking into the accompanying Ramsar Information Sheet (2013) for Lille Vildmose it appears that the main benefit after rewetting is increased carbon sequestration in these restored parts. However, although carbon sequestration increases the main contribution to climate regulation is a reduction of CO2 emissions. Hence, the information sheet needs updating (Barthelmes et al. 2015, Davidson et al. 2019).
Biodiversity
Different methods are used to assess biodiversity. Overall, the results clearly indicate improved conditions for biodiversity in Lille Vildmose, however, some information is limited even though Lille Vildmose is probably one of the best surveyed Natura 2000 areas in Denmark.
Natura 2000 habitat assessments
The Environmental Protection Agency in Denmark has developed a quality assessment tool for species and habitats covered by the two EU directives (Habitats and Birds), which is also used in the Lille Vildmose Natura 2000 area to assess habitat quality.

Fig. 6.1.3. Government assessment of wetland and forests habitat types in the Natura 2000 site in 2004–6 (left) and 2010–11 (right). Blue is high quality habitat, green is good and regarded to meet the assessed EU directive standards. Yellow is moderate, orange bad and red very bad quality. The three last categories are regarded not to meet the EU standards as assessed by the government. Note that a more detailed habitat assessment was undertaken in the 2nd monitoring period. Danish Environment Agency, Miljøstyrelsen.
Data were available in 2004–2006 (before the major restoration activities), in 2010–11 and 2016–17. The results show that the quality of the raised bog habitat (and degraded raised bog, also an EU habitat type) have generally improved between monitoring periods according to the assessment standards used by the Danish Environment Agency (Fig. 6.1.3). Raised (or degraded raised bog) constitutes by far the largest habitat type in Lille Vildmose. The more detailed habitat mapping in 2010–11 shows that especially the lag zone is not in a favorable condition (red color in Fig. 6.1.3), which is related to extensive drainage at the edges of the bog. Overall, the habitat quality has improved between the two monitoring periods.
The assessment in 2016–19 documented that almost 19 km2 raised bog habitat was assessed to be of high or good quality, which is by far the majority of the habitat (92%) and being a result of management interventions. These good quality areas are characterized by a high number of characteristic bog species and a low number of grasses and herbs. An area of about 4 km2 of degraded raised bog is likewise assessed to have maintained its quality and may with time move into a classification as raised bog habitat.
Ramsar assessment
The designation of Lille Vildmose as a Wetland of International Importance (Ramsar site) was based on two criteria: 1) the area contains a large active raised bog with large areas of degraded raised bogs still capable of natural regeneration. Moreover, the climate mitigation argument was added to this criterion. Furthermore, the raised bog habitat qualified because it comprises threatened ecological plant communities. A number of noteworthy species are mentioned in the accompanying Ramsar Information Sheet (Ramsar Convention on Wetlands 2013).

Fig 6.1.4. Showing the active part of the raised bog in Lille Vildmose all-together comprising c. 2,250 ha. The area is valuable for biodiversity although not species rich with a number of threatened and rare plant communities including Sphagnum mosses and Drosera intermedis, Rhynchospora alba and Scheuchzeria palustris. The natural fauna associated with the bog vegetation comprise species such as Curlew (Numenius arquata) and Common crane (Grus grus) (Naturstyrelsen 2013). Moreover, the raised bog contain a significant storage of carbon stock all-together an estimated 4.5 million ton of carbon (Barthelmes et al. 2015). Photo: Tage Burholt.
Biodiversity assessment using the Delphi Method
An assessment of biodiversity was conducted by interviewing key experts using the Delphi method in a MSc project (Bachmore 2018). The questionnaires included assessments on birds, vascular plants and mosses and related to the scientific expert judgements on effects of restoration for selected indicator species within these groups. For 11 out of the 12 species of birds the restoration activities were expected to be “good” or “very good”, in parallel for 6 out 11 vascular plants and 6 of 10 mosses the habitat quality would be expected to be “good”. No species habitats were expected by the key experts to decline in quality.
Evaluation of evidence
Climate
On the one hand area information is detailed thus we have a comprehensive knowledge of the area of the different land use categories (see Tab. 6.1.1, Fig 6.1.1). On the other hand, there are no area specific flux measurements of greenhouse gas emissions or carbon stock changes, which means that the assessment must rely on standard emission factors from the IPCC guidance (IPCC Wetlands Supplement 2014). These standard figures however, are generally used for national inventories and not detailed locality specific studies, which means that there is a large degree of uncertainty in these calculations. Area specific measurements are preferable, however, the proportions of the calculated emission reduction (ton CO2-e ha1 yr1) are not expected to change (Tab. 6.1.1). Flux studies from other drained organic soils in this part of Denmark generally supports these conclusions (Karki et al. 2016, Elsgaard 2018).
Biodiversity
The biodiversity studies range from specific research projects over interviews of scientific key experts to government assessments (verified by Aarhus University) of habitat quality using data on species and structural parameters related to management.
Knowledge of indicator and/or flagship species are also included in Ramsar reporting due to large activities of citizens especially in the last 2–3 decades in the area. Parties to the Ramsar Convention are obliged to fill in a Ramsar Information Sheet (RIS). Thus, the RIS is expected to be used as a monitoring tool updated every sixth years.

Fig 6.1.5. Aerial photo: Before (left) and after (right) restoration of hydrology in the bog restoration areas (dark areas in the second photo below) including restoration of a former lake drained in 1760–70. © Styrelsen for Dataforsyning og Effektivisering.
Synergy
- Conservation of the active raised bog habitat including maintenance of natural hydrological conditions favours biodiversity as well as climate because it provides habitat for an original set of bog species and secure the preservation of the large carbon stock. Moreover, the hollow-hummock-structure of intact active raised bogs has been maintained and restored as well as securing the habitat and the presence of rare bog dependent species.
- The hydrological restoration efforts in Lille Vildmose during the last 10–15 years have reduced the C02eq emission by about 10,500 tons/year. At the same time the benefits for biodiversity in these degraded peatland areas have been positive. This has resulted in a rapid expansion of wetland habitats e.g. transition mires, reedbeds, quaking mires and young birch woodland with a number of characteristic raised bog species being observed.
- Immediately flooding of drained organic soils may favor climate but compromise biodiversity because preparatory activities will delay rewetting but in a number of situations provide the best biodiversity results e.g. in terms of levelling out the ground to prevent sudden flooding and deeper water basins that prevent regrowth of Sphagnum etc.
Acknowledgement
Many thanks to Peter Hahn, Danish Nature Agency, Jacob Palsgaard Andersen, Aage V. Jensen Nature Foundation and Roar Skovlund Poulsen, Aalborg Municipality for providing comments to an earlier draft and Hans Joosten, Greifswald University for additional information. We do also thank Kamilla Harlev Mai, Danish Environment Agency for providing information on the Natura 2000 assessment.
6.2. Restoration of natural landscape hydrology - mire restoration, Sweden
Introduction
This case describes the concerted effort to restore hydrology in 35 mires in Sweden.
Reclaiming wetlands for agriculture has been going on in Sweden for hundreds of years. It has been estimated that about one-fourth of the original wetland area in Sweden has been drained and turned into arable land and grasslands, predominantly between 1850 and 1900 (Löfroth 2001, 2015). The use of wetlands for agriculture reached a maximum in 1920–1950. At the end of this period, about 7,050 km2 were drained (Barthelmes et al. 2015).
Large peat extraction sites were also established in the late 1800s and excavation carried out with varying intensity and later with peaks during the world wars and the energy crisis in the early 1970s. About 100 million m3 peat were extracted between 1980–2012 (Statistiska centralbyrån 2013).
Today the creation of new drainage systems for agriculture or forestry has generally ceased in peatlands (Naturvårdsverket 2014, Löfroth 2015) and new drainage systems are prohibited in parts of Sweden, although dispensation may be given under certain conditions (Naturvårdsverket 2014). Restoration of the effects of former drainage activities has been initiated in a few peatlands in the last decades. This case presents an example of such restoration in the project labelled “Life to ad(d)mire”.
This EU LIFE supported project “Life to ad(d)mire” 2010–2015 is the largest peatland restoration project in Sweden to date, with the aim of restoring degraded mires. Restoration activities were covering a total of approximately 28–30 km2 and with an effect on the hydrology of about 400 km2 of land. Though still a very small fraction of the total peatland area drained in Sweden.
Case
“Life to ad(d)mire” was undertaken in 35 drained and degraded mires in Sweden. The project was funded by the EU Commission and co-funded by the Swedish Environmental Protection Agency and implemented in collaboration with the county administrations. The project restored former hydrological conditions in the mires in existing nature conservation areas.
Restoration interventions were conducted in mires situated within or connected to existing Natura 2000 areas resulting in restored hydrology in the drained mires, removed trees and shrubs in about 18 km2 of overgrown peat and restored 15 ha overgrown wet meadows for renewed haymaking (LIFE 2016).
The objective of these interventions was to enhance the conservation status of habitat types and species habitats targeting in the EU directives and with a special focus on the EU priority habitat of raised bog. A special effort was made to increase areas of Sphagnum regrowth and the quality of the bog plant community and habitats for a number of mire dependent bird species.
Thus, the purpose was essentially the same as in the Danish case of Lille Vildmose i.e. restoration of bog habitat covered by EU legislation (see chapter 6.1.). However, perhaps a difference was that the targeted mires in Sweden were all drained but they had not lost their original plant communities although they were going to change due to drainage and regrowth by invading trees and shrub.

Fig. 6.2.1. Left: General mire regions in Sweden: 1) Mountain mires, 2) Palsa mires, 3) Northern Aapa mire subregion, 4) Central Aapa mire subregion, 5) Soligenous Aapa mires subregion, 6) Southern Aapa mire subregion, 7) raised bog region, and 8) Pine bog-marsh region. Right: Mire areas targeted for project intervention 2010–15 in the restoration project (see also Tab. 6.2.3 for a list of localities). Source: (Gunnarsson & Löfroth 2014, Löfroth 2015, LIFE 2016).
The main interventions were targeting areas affected by drainage ditches and as a result the mire habitat were severely altered and often overgrown with accelerated invasion of trees and shrub making them less suitable or unsuitable as habitats for a number of species depending on open water saturated mire habitat.
The activities included blocking of ditches and construction of dams and thereby retaining water on the mire by using the natural geography as boundaries and banks. Moreover, removal of shrubs and trees were undertaken. A total of 11 habitat types and 6 species listed in the EU Habitat Directive and 14 bird species listed in the Birds Directive were targeted and expected to be promoted by the restoration actions. A main target was to restore hydrological functions of the mire ecosystems (not necessarily towards an expected situation before the particular mire was degraded).
In the final project report to the EU Commission (LIFE 2016) it was stated: “The biggest threat from peatlands in non-favourable conservation status is the climate effects. This is an issue that is rising on the horizon on a lot of levels, both regional and nationally. It is a serious issue and knowledge is the key.” Thus, long term effects from the restoration may include more knowledge on the specific greenhouse gas emissions and increased knowledge on how to restore cost effectively.

Fig 6.2.2. Excavator filling in a drainage ditch to raise the water level in a drained mire. Notice the invasion of trees along the banks of the canal where the ground is higher due to drainage. All-together more than 3,200 dams were established and in total, the project has restored hydrology in more than 2,800 hectares of drained mires, removed trees and shrubs in 1,800 hectares of overgrown wetlands, and restored 15 hectares overgrown wet meadows for renewed haymaking. Source: The EU LIFE to ad(d)mire project 2010–2015. Photo: Länsstyrelsen, Jämtland.
Land tenure and protection status
The land was both privately and state owned (see below).
The mires are covered by Natura 2000 status. Specific mire habitat types such as raised bogs, fens and Aapa mires are EU listed habitats for designation of specific Natura 2000 sites. A number of peatland birds are listed for the designation of EU bird areas often overlapping with the EU habitat areas because these nature types provide key habitats for birds.
Certain mires are covered by National Park or Nature Reserve status and/or have been designated as Wetlands of International Importance (Ramsar sites).
Current land use and management interventions
The drainage and exploitation have been driven first and foremost by agriculture, secondly forestry within or in adjacent areas as well as peat extraction. The 35 sites intervened by the ad(d)mire project have not been cultivated or excavated to any large extent and they are in this respect less exploited than many other Swedish peatland areas.
During the project intervention, different adaptations of restoration methods were used because of differences in peat layer depths and the composition of the mire, different sizes of the width and depth of the ditches and different density of invaded tree species (Tenning 2015, LIFE 2016).
Nature based tourism has been actively promoted as part of the project. Five bird-watching towers and five shelters have been built and walkways have been constructed (Tenning 2015).
Stakeholders
The County administrative boards of the following seven local authorities “Länsstyrelser” were steering the project interventions in their respective counties: Dalarnas, Jämtlands, Jönköpings, Kronobergs, Skåne, Västernorrlands and Östergötlands Län and with the overall responsibility vested in administrative board of Jämtland. The project was supported by the Swedish Environmental Protection Agency.
Dialogues with the landowners were undertaken throughout the project. Directly involved were also owners of neighbouring areas as well as employees at the seven county administrative boards and relevant municipalities. Consultation was undertaken with fish conservation societies, bird watcher clubs, conservation associations and hunting clubs.
A total of 28 water operation permits were approved during the project to secure land owner rights and provide opportunities for compensation after restoration is completed. Furthermore, 13 management plans were revised as part of the project.
Biodiversity
Biodiversity was the main focus of the interventions with the purpose to improve the conservation status of habitat types and species listed in the EU directives. The expected results were improved conditions and a move towards favorable conservation status (LIFE 2016), which is a prerequisite for the financial support of the LIFE contribution from the EU Commission and the overall aim of the Habitat Directive.
Monitoring was undertaken by counties with varying intensity and of different biological target groups before and after restoration at some of the sites. The bird and vegetation monitoring has generally been implemented following national methods and should thus be comparable to other Natura 2000 sites in Sweden. Electro fishing was performed in Stensjöflon (LIFE 2016). Monitoring results from Kronobergs Län, Jämtland and Västernorrland are presented below.

Fig. 6.2.3. Black grouse (Tetrao tetrix). One of the target species for monitoring interventions at the ad(d)mire restorations sites along with other EU listed directive species. Photo: Lars Petersson.
Kronobergs Län (Årshultsmyren, Horsnäsamossen, Hästasjömyren and Tängsjöfly).
Populations of four EU directive species are stable or fluctuating and with indication of increase in some populations although further monitoring needs to verify this (Tab. 6.2.1.). It should be pointed out that the four species in Tab. 6.2.1 are the basis for EU designation of Natura 2000 sites because they are assessed threatened in the EU partly because of deteriorations of habitats and population declines and they have high-quality habitat requirement compared to many other species.
| Breeding species | 2010 before | 2020 after | ||
| SPA listing (EU Birds Directive) | Apr/May | May/Jun | Apr/May | May/Jun |
| Crane (Grus grus) | 3 | 1 | 2 | 11 |
| Golden plover (Pluvialis apricaria) | 2 | 10 | 4 | 8 |
| Black grouse (Tetrao tetrix) | 42 | 5 | 47 | 19 |
| Wood sandpiper (Tringa glareola) | 6 | 3 | 10 | 4 |
Tab. 6.2.1. Breeding species listed in the EU Birds Directive from five restored mire sites in Kronobergs Län. Counts of the populations of the sites were conducted between 25 April and 15 May (Apr/May) and between 25 May and 10 June (May/Jun) in 2010 (before restoration) and 2020 (after restoration). On five occasions in 2010 the number were recorded as unknown for which the relevant numbers for both 2010 and 2015 were removed from the data. Source: Anonymous 2015.
Fig 6.2.4. Example of the development of vegetation in one mire in (Årshultsmyren) in Kronobergs Län. Left: Abundance of typical species in plant communities: The grey bars are before and the red bars after restoration. Right: Density of Sphagnum mosses in 2010 (before) and 2015 (after) restoration. Source: Götbrink (2015).
From monitoring of vascular plants and mosses (Fig. 6.2.4) indications of increase in mire associated plant communities and mosses are detected using grid transects although some monitoring results are mixed.
Jämtland (Tysjöarna, Öjsjömyrarna, Stensundet, Brötarna and Ånnsjön).
At one locality (Tysjöarna) monitored in 2013 (before restoration) and 2017–2018 (after restoration) the results showed significant negative trend of a number of waders including the EU listed species due to flooding of the habitat - number of territories declined with 81% (Råghall 2019). Thus, the high water table has resulted in temporary loss of habitat and thus numbers of breeding and resting mire species crashed. To improve conditions for these waders the water level will need to be lowered.
Västernorrland (Mossaträsk, Stensjöflon, Gideåbergsmyrarna, Sörlappmyran and Prästflon).
Monitoring of birds were conducted at Stensjöflons Nature Reserve in 1983 and 2017. The results are shown in Tab. 6.2.2 for the EU listed bird species.
| Species | 1983 | 2017 |
| Stensjöflon NR | Stensjöflon NR | |
| Whooper swan (Cygnus cygnus) | 0 | 1 (+1)* |
| Crane (Grus grus) | 3 | 2 |
| Golden plover (Pluvialis apricaria) | (1) | 2 |
| Wood sandpiper (Tringa glareola) | 16 | 11 |
Tab. 6.2.2. Birds listed in the EU Bird Directive monitored in Stensjöflon Nature Reserve in 1983 (long before restoration) and in 2017 (after restoration). Numbers correspond to territories. * One additional territory at Runflon not monitored in 1983. Source: Bader (2019).
The results show that none of the EU listed species has been lost from the site and that a Whopper swan have colonized the site in the meantime. Examination of changes in the overall bird community between 1983 and 2017–2018 may lead to the overall conclusion that the loss of populations has been halted and future monitoring will reveal if this is followed by a population increase (Bader 2019). However, the numbers of Wood sandpipers were lower after restoration. Eventually, the mire habitats can be restored and become attractive, however, it is up to the species themselves to recolonize their former habitats.
Photo documentation has been extensively used to show before and after situations. Many of these photos clearly document change in hydrology and habitat in favor of mire habitat (see Fig. 6.2.5, 6.2.6, 6.2.7).
Climate
Greenhouse gas fluxes were not measured in the restored mires. Hence there are no site-specific information on effects from restoration on the climate.
A desk-top calculation has been conducted as part of this report, based on the standard emission factors provided by the IPCC guidelines (IPCC 2014) and guidance in Joosten et al. (2018) and areas provided in Tab. 6.2.3. We have not distinguished between nutrient rich or nutrient poor peatlands in the calculations because some mires may be intermediate or comprise both rich and poor parts. Moreover, we have used rather conservative values (see proposed values in Joosten et al. 2018).
Thus, during the project almost 30 km2 of mires were restored at 28 sites (LIFE 2016). The rough calculations mentioned above provides an overall estimate of a reduced emission of 18,779 ton CO2-e per year using an average Gross Warming Potential (CO2-e ha per year) of 11 for boreal peatlands, and 9 for temperate peatlands following recommendations in the IPCC 2013 Wetlands Supplement (IPCC 2014) and Joosten et al. (2018). This figure is uncertain and provides a rough indication of the climate effect from the restoration activities as a result of the ad(d)mire project activities (see chapter 2.2. for further details).
However, the restored area may actually have been larger as the hydrological effect of the restoration was expected to cover some 400 km2. If this is correct the reduction may be higher.
| Locality (NATURA 2000 site) | Restored hydrology (area, ha) | Emission reduction GWP CO2-e ha per year | Total GWP CO2-e ha per year | Measures (no. of dams in blocked ditches) |
| Brötarna | 1,520 | 11 | 5,016 | 11,650 |
| Tysjöarna | 115 | 11 | 1,265 | 140 |
| Blekbäcken, Stensundet | 48 | 11 | 528 | 114 |
| Ånnsjön | 77.5 | 11 | 853 | 67 |
| Öjsjömyrarna | 51 | 11 | 561 | 42 |
| Fjällmossen (i Kolmården) | 29 | 9 | 261 | 47 |
| Bredsjömossen | 3.5 | 9 | 32 | 5 |
| Kärnskogsmossen | 15 | 9 | 135 | 16 |
| Haftahedarna | 26 | 9 | 234 | 15 |
| Koppången | 46 | 11 | 506 | 15 |
| Blåbergsåsflyten | 10 | 11 | 110 | 11 |
| Komosse | 42 | 9 | 378 | 50 |
| Store Mosse NP | 336 | 9 | 3,024 | 170 |
| Store Mosse, Anderstorp | 308 | 9 | 2,772 | 1,000 |
| Årshultsmyren | 91 | 9 | 819 | 6 |
| Horsnäsamossen | 56 | 9 | 504 | 24 |
| Hästasjömyren | 17 | 9 | 153 | 26 |
| Taglamyren | 30 | 9 | 270 | 29 |
| Tängsjöfly | 1 | 9 | 9 | 22 |
| Mossaträsk | 31 | 11 | 341 | 95 |
| Stensjöflon | 28 | 11 | 308 | 52 |
| Gideåbergsmyrarna | 17 | 11 | 187 | 21 |
| Sör-lappmyran | 31 | 11 | 341 | 100 |
| Djurholmamossen | 3.5 | 9 | 32 | 3 |
| Lya Ljunghed och Älemossen | 7 | 9 | 63 | 10 |
| Söderåsen | 6 | 9 | 54 | 15 |
| Traneröds mosse | 0.5 | 9 | 5 | - |
| Dagstorps mosse | 2 | 9 | 18 | - |
Tab. 6.2.3. Peatland areas (mires) with restored hydrology by the ad(d)mire project. The estimate of the reduced emission is based on Joosten et al. (2018) and envisaged to be rather conservative estimates.
Abiotic parameters such as ground water table and quality were monitored. These water samples were taken before, during and after the restorations at some of the sites but have not been examined during this study.
The ad(d)mire project covers a small fraction of the total area of organic soils in Sweden estimated at c. 85,000 km². About 18% corresponding to approximately 15,500 km² has been drained (Barthelmes et al. 2015 and references therein). Thus the ad(d)mire project covers just 0.2%. The resulting CO2 emission from the total area of drained peat soils has been calculated to be 10.6 Mt CO2 per year or 23% of the total Swedish CO2 emissions (without LULUCF, Barthelmes et al. 2015).
Evaluation of evidence
Climate
Greenhouse gas fluxes were not measured. It is however stated in the project report (LIFE 2016) that the interventions are assumed to have a positive climate impact. By using figures from the IPCC 2013 Supplement: Wetlands (IPCC 2014) and in Joosten et al. (2018) and data on size of area rewetted and general soil type a preliminary attempt have been conducted during this project to calculate a total emission reduction (Tab. 6.2.3). This method is used during national reporting to the IPCC and is also used in the Lille Vildmose case in Denmark (see chapter 6.1, Barthelmes et al. 2015 for justification) and is a very rough alternative when there is no site-specific flux data.
Biodiversity
Overall biodiversity monitoring has been undertaken in some counties and targeting selected species and species groups and in particular species listed in the EU Birds and Habitats Directives. For certain localities monitoring were undertaken before and after restoration as presented in the biodiversity section above. Inventories were conducted by local experts and coordinated by the county administrations.
The procedure developed by the Swedish Environmental Protection Agency (SEPA) for vegetation and bird monitoring in Natura 2000 areas was expected to be followed and the data reported to a national Natura 2000 database. No common biodiversity analysis or testing related to the restoration projects overall has been conducted.
Arial photos have been a very important and effective means of verification of habitat changes before and after restoration. Although the level of detail is limited this serie of photo documentation is convincing in their demonstration of change. The visual effects of the interventions are obvious and as such has been a successful documentation tool by the project. However, combination with fieldwork on the ground is needed.

Fig. 6.2.5. Photo documentation. Monitoring Årshultsmyren 2010 (before) and 2015 (after) restoration in terms of the removal of trees and shrubs (see also Tab. 6.2.3). Direct restoration of a higher water table by establishing dykes was implemented in other parts of Årshultsmyren. Photo: Bergslagsbild.

Fig. 6.2.6. Photo documentation. Aerial monitoring in Anderstorp Stormosse in 2010 (before), 2015 (right after) and in 2020 (five years after) restoration. A high-water level was restored and trees and shrubs removed (see also Tab. 6.2.3). Photo: Bergslagsbild.

Fig. 6.2.7. Photo documentation. Aerial monitoring in Store Mosse in 2010 (before), 2015 (right after) and in 2020 (five years after) restoration. A water level near ground level was established by lowering dry ridges. Trees and shrubs were removed. The line in the middle is a railway. (See also Tab. 6.2.3). Photo: Bergslagsbild.
Synergy
This is an example of synergy between biodiversity conservation and climate change mitigation most effectively documented by aerial photographs and combined with general knowledge from research on these two topics (see chapter 2.2. and 3). The implemented biological monitoring schemes provide clear indications that species listed in the annexes of the EU directives are improving at some monitored sites. The general knowledge of the effects on rewetting peatlands provide confidence in that emissions are reduced at the rewetted sites although site specific flux measurements have not been undertaken.
The proportions of such a reduction of the CO2 emission from the all-together 35 restoration projects are not precisely known and would require site-specific flux measurements. A rough calculation using the IPCC standard figures for wetlands came to an indication of 19,000 CO2-e ha per year based on an assumption that 28–30 km2 of mire has been rewetted. However, this area may be larger as the hydrological effect of the restoration covered 400 km2. If this is correct the reduction may be higher.
By rewetting the presumably large carbon stocks at each mire is being effectively preserved. No measures are known to the authors of the depth of each individual mire and hence the total carbon stock at each site.
Further documentation of the results from the restoration activities in terms of both biodiversity and climate will be interesting.
Acknowledgement
Many thanks to Jenny Lonnstad and Michael Löfroth, Swedish Environmental Protection Agency for facilitating contact and to Magnus Strindell, Länsstyrelsen i Kronobergs Län and Henrik Gustafsson and Kristofer Paulsson, Länsstyrelsen i Jönköpings Län and Kristin Lindström, Länsstyrelsen Västernorrland for facilitating access to information and again to Kristofer Paulsson for providing comments to an earlier draft and access to aerial photos.
6.3. Palsa mires – a threatened sub-arctic habitat, Norway
Introduction
Due to rapid change in climate, palsa mires are rapidly disappearing. Palsa mires are a habitat type generally protected as well as designated as Emerald sites under the Bern Convention and in EU Natura 2000 sites. If the current rate of climate change continues the habitat may be lost from the Nordic countries within decades. There seem to be no imediate management opportunities other than to reduce emissions at the global level by negotiating ambitious targets and to accelerate implementation efforts. Palsa mires may also be seen as an illustrative example at the habitat level of climatic driven change and degradation of biodiversity and certain habitat types caused by these rapid climatic changes (IPBES 2019).
Palsa mires are wetland and peatland types formed by a mosaic of permafrost mounds and plateaus, ponds and mire sections without permafrost. They occur in regions with discontinuous or sporadic permafrost and have a disrupted distribution across the northern boreal region (Fig. 6.3.1). The distribution of palsa mires in the Nordic countries includes the northern and central highlands of Norway, and northern Finland and Sweden as well as a single occurrence in Iceland. The rapid changes in this entire habitat type are complex but related to climate change including increased temperature and change in precipitation patterns (Hofgaard 2003). The effects seen on Palsa mires are novel examples of changes happening when permafrost is thawing in the northern boreal region throughout the Northern hemisphere.
Palsa mires are peatland habitats, which stores carbon and hosts species adapted to boreal and subarctic environments. An increase in carbon fluxes to the atmosphere (especially in methane, CH4) from thawing permafrost soils with high content of organic content may turn the subarctic region to a net carbon source (Koven et al. 2011, Borge et al. 2017), although the full consequences are not clear.
In total, the northern permafrost region (i.e. with permanently frozen soils) may contain perhaps 1300 Pg (petagram/billion tonnes) of organic carbon, of which the majority occurs in permanently frozen soils and deposits. These 1300 Pg of organic carbon account for approximately 40% of the estimated global belowground organic carbon pool (Tarnocai et al. 2009, Hugelius et al. 2014) which again corresponds to a significant proportion of the carbon currently in the atmosphere (Jones et al. 2017). Moreover, significant emissions of N2O have been observed in peat plateaus in northwestern Russia and in palsa mires in Finland (Borge et al. 2017, Marushchak et al. 2011), highlighting the active role of palsa mires in the coupled carbon and nitrogen cycles in these regions.
In Norway, palsa mires are restricted to the northeastern part and a few areas in the montane area of central Norway, at elevations between 700 to 1400 m above sea, and where mean annual temperature is generally below 1˚C. Palsa mires are characterized by mosaic of areas of permanently frozen hummocks and plateaus intermixed with peat without permafrost and ponds created by former, but now disintegrated, palsa formations.
Large variations in temperature and precipitation between different palsa mires and local changes in e.g. snow depth and water balance (Martin et al. 2019) indicate a very sensitive balance in the formation and persistence of palsa mires and thus the effect from climatic changes at all geographical levels on their physical and chemical composition as well as on their corresponding biological communities.
Case
In order to follow the effects of climate change on palsa mires the Norwegian Institute for Natural Research (NINA), financed by the Norwegian Environment Agency, runs a monitoring program. The findings from this may actually provide indications of the future development of the much larger circumpolar permafrost areas that today are also becoming affected by climate change.
The Norwegian monitoring program was initiated in 2004, designed to reveal changes in these climate-sensitive ecosystems. The study area includes six permafrost areas from Finnmark in northern Norway to Dovre in south-central Norway close to the Barents Sea and the Atlantic, at the western fringe of the North-Eurasian permafrost region.
The monitoring program include ecosystem components such as soil structures and biological communities, and it monitors the rate at which changes to this landscape occur (Hofgaard 2019). The formation of Nordic palsa mires coincide with cool periods in the Nordic history after the last ice age. Palsas became widespread in Europe when climate cooled at 3000–2500 BP and active periods in permafrost formation seem to have occurred around 2500–1900 BP in Fennoscandia and during the so-called Little Ice Age 700–100 BP although based on limited datings.
In this case study we relate the findings from the Norwegian monitoring to studies in Alaska, Canada (e.g. Payette et al. 2004), Finland and Russia. These studies have looked into the long-term development of palsa mires using chronosequence studies, and discus the effects of the thawing of permafrost peatlands in the boreal zone (Jones et al. 2017, Olvmo et al. 2020).
Box 1.
Palsa mires are classified in the EUNIS habitat classification (Council of Europe 2015) relevant to the Bern Convention and the EU habitat directive. Distinct types are recognized (modified after Joosten et al. 2015) and all dependent on permafrost in the subarctic discontinuous permafrost zone.
Classification of Palsa mires in the EUNIS Classification system:
Mires of the subarctic and northern boreal regions formed by elevated frozen mounds or ridges (palsas), 0.5 to 8 m high and up to 50 m in diameter, interspersed wet hollows of similar area. Palsa mires are distributed in the discontinuous permafrost zone of Iceland, northern Fennoscandia and arctic Russia and North America, in areas experiencing subzero temperatures for at least 200 days per year.
Four types of palsa mires (Joosten et al. 2015):
- With permafrost in mounds and plateaus (flat, individual mounds in fen matrix, without polygonal structure) – Flat and domed palsa mires,
- With permafrost in strings (sloping, permafrost underlain strings alternating with unfrozen flarks – Ribbed palsa mires,
- With irregular polygonal surface structure – Polygonal flat palsa mires
Permafrost is by the International Permafrost Association (IPA, 2014) defined as “ground (soil or rock and include ice or organic material) that remains at or below 0 °C for at least two consecutive years”. The term palsa originates from the Finnish and Saami languages and mean peat tussock or mound in peatlands (Hofgaard 2003)

Fig. 6.3.1. The distribution of continuous and discontinuous permafrost in the northern hemisphere covering the Nordic countries, and Russia, Canada and United States. This case study from Norway focuses on Palsa mires in the discontinuous areas of permafrost (source: International Permafrost Association 1996 and Hofgaard 2019).
Finland de facto have an obligation to conserve this habitat. Norway is a member of the Bern Convention on the Conservation of European Wildlife and Natural Habitats.
Moreover, some palsa mires are covered by a status as wetlands of international importance under the Ramsar Convention. Some palsa mires do have National park status e.g. Dovrefjell-Sunndalsfjella National Park and are Nature Reserves.
Current land use and management
Because palsa mires are located in the subarctic zone they are generally outside the zone for commercial forestry or agriculture. Thus, extensive drainage of peatlands as seen in the southern lowland part of Norway and other parts in the Nordic countries has not – at least not to a large extent - taken place in the regions with Palsa mires. Some palsa mires have disappeared due to creation of water reservoirs for power plants.
Land use comprise grazing and berry picking by domestic sheep and semi-domestic reindeers. In contrast to many other peatland regions peat extraction for fuel is of no or low importance in Palsa regions. The increased use of ATWs might be a future threat, as tracks develop into drainage channels (A. Hofgaard pers. com).
A review by Markkula et al. (2019) suggested that the most profound negative impacts from global warming on traditional livelihoods and indigenous peoples and local communities will be in the regions on Palsa mire and fell ecosystems: “Consequently, changes in ecosystems may erode cultural meanings, stories, memories and traditional knowledge attached to them and affect the nature-based traditional livelihoods.”
Overall, there is little scientific doubt that the primary driver of the deterioration of palsa mires is climate change and land use changes are secondary (Hofgaard 2019).
Stakeholders
Climate change is a global responsibility as greenhouse gas emissions rapidly mix in the atmosphere. Thus, their deteriorations are the unfortunate result of the accumulated emissions especially by the developed world.
At the local level, communities and landowners living close to these peatlands have used them at near sustainable levels for e.g. berries, reindeer herding and hiking etc.
Tourists are attracted to some sites including National Parks and Emerald Network sites, which are visited by the outdoor community including international tourists.
Climate
Monitoring of palsa mires in Norway does not include Green House Gas flux measurements but does involve data collection on climate parameters such as temperature, precipitation etc. at meteorological stations. In order to assess the effects on climate other studies have been included at a more generic level.
Recent warming at high latitudes has accelerated permafrost thawing in northern peatlands (Hofgaard et al. 2019, Payette et al., 2004; Camill, 2014). This may release CO2 from formerly frozen peat deposits by increasing decomposition. It may also increase emissions of CH4 because in many cases the peat surface becomes inundated (Johnston et al., 2014, Klapstein et al., 2014, Jones et al. 2017). However, although carbon cycles are complex and carbon is continually accumulating in mires, it may take hundreds to thousands of years to restore the carbon stock present prior to permafrost thaw (Jones et al. 2017). Moreover, no less than 40–90% of continuous and discontinuous permafrost area may thaw by the end of the century (Lawrence & Slater 2005).
Thus, permafrost thaw may lead to increased atmospheric warming depending on whether carbon losses from thawing soils outweigh carbon gains in soils and vegetation by increased vegetation growth. This will certainly vary as well, dependent on the specific localities. However, several recent studies predict net carbon loss (Koven et al., 2011; Schuur et al., 2015).
Since the 1950s, the area covered by Palsas mires in the Finnmark in northern Norway has steadily decreased through lateral erosion and the immediate formation of thermokarst ponds when the Palsa mounds themselves collapses (Fig. 6.3.2 and 6.3.3). A study at four areas showed a total decrease in area between 33 and 71% (Borge et al. 2017). In this period air temperatures increased by 1.0–1.5 ˚C in these areas and there was a significant increase in precipitation (both rain and snow).
Jones et al. (2017) found in their study in Alaska that it can take multiple centuries to millennia for a site to recover its pre-thaw carbon stocks by accumulation of new organic material via photosynthesis. The duration of recovery depends on the amount of carbon that accumulated prior to thawing. This means that older peatlands with deep peat layers will take longer to recover prethaw carbon stocks, whereas younger peatlands will exceed prethaw stocks in a matter of centuries. It was concluded that the loss of sporadic and discontinuous permafrost by 2100 could result in a loss of up to 24 Pg of deep carbon from permafrost peatlands and that this zone may contain 85.1 Pg of carbon (Tarnocai et al., 2009; Hugelius et al., 2014).
Looking at the entire permafrost zone in the northern hemisphere large quantities of organic carbon are stored in permafrost in the Arctic and sub-Arctic region. Global climate change will induce environmental changes that accelerate the microbial breakdown of organic carbon and the release of the greenhouse gases. This feedback may accelerate climate change, but the magnitude and timing of greenhouse gas emission from these regions and their net impact on climate are still subject to great uncertainty. Although current evidence may suggest a gradual and prolonged release of greenhouse gas emissions with a warming climate many aspects of permafrost carbon dynamics remain unresolved (Schuur et al. 2015).
Several studies indicate that if all permafrost peatlands follow a trend of substantial carbon loss, these landscapes may after an initial loss shift back to net carbon sinks as mires develop and offset the initial losses from thaw immediately, after centuries or millennia, depending on hydrological settings etc. (Payette et al. 2004, Jones et al. 2017).
Biodiversity
No single species seems to be confined to Palsa mires. However, some plant assemblages and vegetation successions are typical in permafrost landscapes.
Such biological communities include mosses such as Dicranum elongatum and Polytrichum strictum and Sphagnum species such as S. lindbergii or S. riparium and Eriophorum communities in flarks near to Palsas. Brown-moss (Sphagnum fuscum) and Carex communities are more common in wet habitats further from Palsas (Oksanen 2005). Moreover, there are unique lichen communities on Palsas. Differences between regions is observed, but Palsa vegetation is rather uniform over the whole of Europe (Oksanen 2005). Palsa mires in Scandinavia form important breeding sites for migratory waterbirds including low-density species such as the Broad-billed sandpiper (Limicola falcinellus) (Hofgaard 2003).
Recent formation of apparently temporary Palsas has also occurred in some areas (Hofgaard 2019) but not at any degree to counteract the overall deteriorations seen. Thus, the decay of palsa mires have probably amplified over the past 50–60 years. Rapid changes in extent seems to be caused by wetter, warmer and shorter winters since the mid-1950s and accelerating in the last decades (Hofgaard 2019). In a study by Olvmo et al. (2020) analyzing meteorological data since the 1880s they showed that average annual temperature conditions for palsa mires generally have been unfavorable for more than a century and annual average precipitation may have been unfavorable since the 1940s.
The loss of habitat is expected to continue, most likely at a higher rate than today (Hofgaard 2019, Olvmo et al. 2020) but even if the present-day loss rates continue in the future, palsa mires will largely disappear in northern Norway in the course of the 21st century.
Evaluation of evidence
Climate
NINA reports used data available from the Norwegian Meterological Institute (Hofgaard 2019, Hofgaard et al. 2020) on air temperature, soil temperature, precipitation, snow depth all at 5-year intervals. Data on greenhouse gas fluxes are not collected at the Norwegian study areas. Other studies with flux measurements in the subarctic are included instead. The carbon cycle is complex and as the discussion in the climate section above indicates, the scientific evidence is ambiguous and depends on locations and parameters and time scale studies etc.
Biodiversity
Palsa mires have been regularly monitored in Norway at selected sites over the last 15 years and the results are presented in monitoring reports from NINA. It includes parameters on land cover type (7 variables) bottom layer (6 variables) field layer (8 variables) shrub layer (3 variables) thawing depth, Palsa heights above surrounding mire, all at five years interval. Moreover, extensive photo documentation has been undertaken (see examples in the following).

Fig. 6.3.2. Monitoring of the development of palsa mires in Norway from 2004–2018. Within this short timeframe, the permafrost induced Palsas has disappeared due to climate change. Source: Hofgaard (2019), Norwegian Institute for Nature Research (NINA).

Fig. 6.3.3. Palsa mires development in Norway from 2004–2018. Within this short timeframe the permafrost induced Palsas has disappeared due to climate change. Source: Annika Hofgaard (2019), Norwegian Institute for Nature Research (NINA).
Synergy
Mitigation of climate change is the only way to rescue palsa mires and its associated biological communities in Norway and in the other Nordic countries where palsa mires occur. Climate change mitigation may at the same time prevent a burst of CH4 emissions when permafrost thaw and the overall CO2 balances are also uncertain all-together providing an additional big risk to uncontrolled complex climatic feedback loops to which our knowledge is still limited.
Acknowledgement
We are grateful to Anika Hofgaard from Norwegian Institute for Nature Research (NINA) for her assistance with relevant literature and valuable comments on an earlier draft; Kjell Tore Hansen and Vibeke Husby the Norwegian Environment Agency for information about Palsa mires.
6.4. Future land use options in peatlands with abandoned forestry, Finland
Introduction
The focus of this case is peatlands in boreal Finland drained for timber production since the 1960s and mostly during the 1970s, but now with a post-drainage tree growth that has been considered too low for profitable forestry. Thus, large peatland areas in boreal Finland are no longer productive in a commercial perspective unless new considerable investments in drainage are raised and make commercial forestry attractive again. Moreover, global concern over biodiversity loss and climate change has increased since the 1970s and decision-making will now reflect such environmental and societal concerns as well.
Nearly 70% of the peatlands in Finland have been used for forestry, agriculture, peat excavation or they have been flooded (Lindholm & Heikkilä 2017). The largest user is the forest sector and more than half of the original peatland area in Finland of approximately 100,000 km2 has been drained (Finnish Forest Research Institute 2014) to increase tree growth and facilitate forestry. However, about 20% of the drained peatland area today is commercially low-productive and may not produce timber values at a profitable scale (LIFE 2018). These abandoned and drained peatlands for forestry contribute to pollution of watercourses as well from release of nutrients and they may act as emitters of greenhouse gasses due to their drainage (Tolvannen & Parviainen 2015, LIFE 2018, Juutinen et al. 2020).
Hence, it is relevant to ask what the future and best management scenarios are for these formerly utilized peatland areas? Several different land use options exists, which have been proposed and intensively studied by research teams (see e.g. Jutinen et al. 2020, Tolvanen et al. 2020) in connection to the LifePeatLandUse project supported by EU LIFE funds. Here the different land-use scenarios were: “1) current use, 2) tree biomass harvesting, 3) intensive forestry, 4) ecological restoration, 5) peat production, 6) peat production and reforestation, and 7) peat production and re-wetting” (LIFE 2018).
This case is based on the findings of these studies. The various options call for prioritization on a regional geographical scale with the mentioned opportunity to consider broader society demands (Juutinen et al. 2020).
The utilization of mires in Finland has been more intensive than in most other Northern countries. Especially forestry and in other cases peat extraction or agriculture have altered mire habitats throughout Finland most intensively in the south (Lindholm & Heikkilä 2017). Drainage of large areas of active peatlands have been implemented first and foremost to improve timber growth often with the use of fertilizer. Today drainage in the remaining pristine mires is almost excluded and drainage activities are now largely directed to the maintenance of existing drainage systems.
Seen from an energy production perspective Finland has been responsible for almost 60% of the annual peat use in the EU for energy (World Energy Council, 2013).
Case
Originally, one third of Finland has been covered by mires (Lindholm & Heikkia 2017). Today, commercially low-productivity peatlands cover more than 8000 km2 drained for forestry. The sites were initially mostly treeless or very sparsely tree fens or nutrient poor transitional pine mires in the Aapa mire region, or pine bogs in the south and mid boreal Finland (Penttilä et al. 2015). Fig. 6.4.1 shows the distribution of peatlands in Finland and their drainage status (Juutinen et al. 2020).

Fig. 6.4.1. Distribution of drained and undrained peatlands and peat production sites in Finland as well as one of the LifePeatUse project study areas (LIFE 2018). The studies analyzed seven options for future land use in these formerly drained low-productive peatlands (Juutinen et al. 2020). License no. 4982070719782. Ecological Economics, Elsvier.
Land tenure and protection status
Peatlands are both state and private owned and with a marked geographical difference. In the north the vast area is state owned while in the south most peatlands are private land.
A Finnish Government Program on the sustainable use of mires and peatlands (Ministry of Agriculture and Forestry, 2012) states that all actions that considerably change the function of peatlands, such as peat production, should be allocated to drained or otherwise degraded peatlands. However, in a regional land-use planning process the suitability of these areas for production needs to be carefully considered (Juutinen et al. 2019, Juutinen et al. 2020). Drainage for forestry or peat mining is not supported by the state any longer (J. Ilmonen pers. comm.).
The first mires were protected in Finland in 1956 and the first mire conservation plan was made in 1960 when drainage of mires for forestry expanded enormously (Lindholm & Heikkilä 2017). In the 1990s almost all mires covered with a national conservation status were included in Finnish Natura 2000 network and a number of peatland additional sites were included as well. The habitat quality of these areas are according to the EU regulations secured and with a demand to restore their favorable conservation status in the longer-term. The total area of protected mires in Finland is approximately 12,000 km2 (Lindholm & Heikkilä 2017 and references therein).
The vast majority of peatlands, however, have no protection status. Some recent projects aim at restoring drained former forest production sites for biodiversity and climate regulation e.g. the Hydrology LIFE running in Finland 2017–2023: www.metsa.fi/en/project/hydrology-life/. Although of high importance to biodiversity these constitute a proportionally small area of the commercially low productivity peatlands under investigation in this case.
Current land use and management
State and private forest owners have since the 1960s invested in more than 50,000 km2 of peatland to promote tree growth, which have involved drainage (Juutinen et al. 2020). The annual tree harvesting demand is estimated to increase from about 60 to over 80 million m3 in the future and a large proportion of this increase is expected to come from peatlands (National Forestry Accounting plan for Finland, 2018).
The area of commercially low-productive peatlands with abandoned forestry comprises about 8,000 km2 of the estimated total of more than 50,000 km2 (Juutinen et al. 2020 and references therein). About 80% of these commercially low-productive peatlands are nutrient poor corresponding to about 6,000 km2, whereas the remaining 2,0000 km2 may be nutrient rich but are less commercially attractive for other reasons including nutrient imbalance or unfavorable structure of the surface peat (Juutinen et al. 2020).
There are different interests and opportunities for the future management of these peatlands depending on e.g. the condition of a site, stakeholders and their interest and the specific history of a particular area as well as a growing demand for restoring peatlands due to e.g. international environmental concerns and commitments. As mentioned, the importance of timber production in peatlands is also growing due to the ongoing development of bio-economy (including wood pellets and tile), with an articulated aim at reducing the use of fossil fuels (Juutinen et al. 2019). But with new EU policies including a Green Deal and a Biodiversity Strategy for 2030 (EU Commission 2020) this may change the course in many of the EU countries.
Earlier aims by the government were to reduce the use of energy peat by 2025 (Ministry of Economic Affairs and Employment of Finland, 2013). However, the latest long-term energy and climate strategy does not mention reductions (Juutinen et al. 2020). In contrast peat extraction was proposed to be phased out after finishing the use of fossil fuels (Ministry of Economic Affairs and Employment of Finland, 2014). New energy peat extraction sites were therefore recently likely to be established in the near-future. However, as mentioned this may change once again due to new policies from the EU in 2020 and new priorities in the Finnish Government.
Juutinen et al. (2020) presented the following elaborated scenarios for future land use of the commercially low-productive boreal peatlands:
1. No Action “Low-productive drained stands were left unmanaged, and the future tree growth and stand development were simulated without any human intervention (i.e. no harvesting and no ditch network maintenance operations). It was expected that the stand will gradually rewet by itself and develop towards a pristine-like peatland ecosystem.”
2. Bioenergy. “At the onset of the simulation current tree biomass including small-diameter trees were harvested for energy, except that app. 20 trees per hectare were left as retention trees (less than 15 m3 ha -1) Accordingly, the NPV [Net Present Value] of timber production consisted of the net revenues from this energy wood harvesting. After harvesting, the natural regeneration of the new stands and their development were simulated assuming the most likely amount and tree-species distribution for each site type, while the water table was expected to gradually rise due to the slow deterioration of ditches that were left without maintenance.”
3. Forestry. “Typical stand management measures, i.e. repeated fertilizations, ditch-network maintenance, efficient site preparation and artificial regeneration by seeding or planting were applied for this option. Forest management was focused on producing timber (the harvest is mostly pulpwood but also saw logs are obtained if the minimum diameter at breast height (DBH) criteria for saw log dimensions are met).”
4. Ecological restoration. “Energy wood harvesting was executed at the onset of the simulation. Stands with current stem volume less than about 7 m3 ha -1 were left growing onsite in order to mimic the sparse stands that are a common feature of natural peatlands. After harvesting the trees, the ditches were actively filled and the stand was assumed to rewet rapidly.”
5. Peat extraction. “In the stands suitable for peat extraction (i.e. average peat layer at least 1.5 m thick) the current tree stand was harvested for energy. Then the peat was extracted during the next 21–47 years after which a birch stand was assumed to be naturally generated on the cutaway sites.”
6. Peat extraction and reforestation. “This option is identical to Peat extraction, but the stands were assumed to be actively managed after the peat extraction period (21–47 years) according to two alternatives a) ash-fertilization, natural regeneration of trees, tending of a sapling stand, pre-commercial thinning and one intermediate thinning, or b) ash-fertilization and natural regeneration.”
7. Peat extraction and rewetting. “Identical procedure to the two previous options with regard to the treatment of the current tree stand and peat extraction. However, after the peat extraction period (21–47 years) the stands were rewetted in order to provide wetlands.”
Further details and descriptions of these scenarios are also found in the references of Juutinen et al. (2019, 2020).
Stakeholders
Regional councils are responsible for drawing up regional plans, which set out the framework for local land-use planning. Key stakeholders are land owners, municipalities, regions including the Regional Center for Economic Development, peat production companies, The Central Union of Agricultural Producers and Forest Owners, The Finnish Association for Nature Conservation and the regional Councils as well as Metsähallitus with its two main tasks to manage most of the protected areas of Finland and to supply wood to the country's forest industry.
Land use planning is sensitive, because it is related to vested interests and various aspects of sustainability. A demonstration project using the so-called YODA tool, coordinated by the Natural Resources Institute in Finland (Luke), has provided useful results in decision-making processes (Kurttila et al. 2020). Thus, in a demonstration project (initiated in 2016) decision-making meetings with stakeholders eventually identified 52 out of 99 demonstration sites where it turned out that stakeholders would be able to leave peatland sites for conservation and accept the non-use of these peatland areas for energy production. Key to this process was planning at large geographical levels creating possibilities for prioritizing and set targets for many sites at a time depending on both local and international factors.
Climate
Analysis of the greenhouse gas fluxes in the drained peatlands have been undertaken by Ojanen et al. (2010). Moreover, the long-term effect of fertilization is presented in Ojanen et al. (2018). This study presents data applicable for estimating soil–atmosphere GHG fluxes for boreal forestry-drained peatlands. It is worth noting that these peatlands may have lost their ability to sequester carbon into the soil (Juutinen et al. 2020).
CO2: Drainage of peatlands for forestry usually leads to CO2 net emission from soil due to decomposition of peat. Thus water table depth is important for explaining variation in CO2 fluxes between sites and mean water table depth significantly explained the between-site variation in CO2 fluxes (Ojanen et al. 2010). Fertilization has been found to increase decomposition rates and thus CO2 emissions compared to merely drained sites, thus the drained and fertilized sites show higher CO2 emissions (Ojanen et al. 2018). Rewetted sites show reduced CO2 emissions in all regions and for all trophic levels. Ojanen et al. (2010) found that annual soil CO2 emission can be reliably estimated by determining CO2 emission (again dependent on the water table) at summer mean soil temperature and almost totally explained by soil respiration.
CH4: Water table depth is also key for explaining variation in CH4 fluxes (as for CO2) and emission increases with increasing water table above ground surface. Well-drained sites are CH4 sinks, but a considerable share of the forestry drained peatlands still emits methane because of high mean water table (Ojanen et al. 2010) although CH4 emissions from many of the drained peatlands were generally measured to be low. Fertilization effects on CH4 emissions were small at most sites (Ojanen et al. 2018). In their flux studies in the Aapa mire region Penttilä et al. (2015) found high CH4 emissions from blocked or filled ditches of restored mesotrophic and oligotrophic sites, and other wet surfaces. Interesting low CH4 fluxes were detected from hummock surfaces at all sites and in pine bogs in the south emissions were detected only from the ditches.
N2O: Again, water table depth is correlated to N2O fluxes. Drained peatlands are potential sources of N2O emissions. However, N2O flux was best explained by peat CN ratio (Ojanen et al. 2010). In the majority of the forestry-drained peatlands N2O emissions are low (Ojanen et al. 2010, 2018) and differ from N2O emissions from agricultural drained peatlands by a more linear negative CN ratio relationship. Fertilization effects on the N2O emissions were small at most sites (Ojanen et al. 2018) and fertilization did not seem to induce a risk of N2O emissions (Ojanen et al. 2010). Due to a correlation of N2O flux with summer mean air temperature and water table depth inter annual variation in fluxes is likely.

Fig. 6.4.2. Low productive peatland site measuring greenhouse gas fluxes in the field at Tolkansuo, Vaala in the Aapa mire region. Source: Poster presentation on “Restoration of low-productive, forestry-drained peatlands - impacts on CO2 and CH4 fluxes” Penttilä et al. (2015). Natura Resources Institute Finland (Luke).
At the more general level the results from the Finnish studies showed that most low-productive peatlands are carbon neutral or carbon sinks (Juutinen et al. 2020). The explanation is that most are slow growing and nutrient-poor pine fens often with a water table, which has naturally increased over the years after drainage up to this day (Juutinen et al. 2020).
Following the land use options described earlier: Option 2 involving wood harvested for bioenergy this option did favor climate mitigation the most according to the modelled scenarios because the water table will raise additionally when trees are removed as the trees contribute to dry up the peatlands but not in a way that will result in large bursts of CH4.
Option 4 involving ecological restoration was considered to initiate a process towards greenhouse gas exchange as it has been prior to drainage and afforestation. The initial effect may be climate warming due to increased CH4 emissions and reduced tree growth. A climate-cooling effect will however be expected in the long-term due to the halt of CO2 emissions (Juutinen et al. 2020). Due to the initial release of CH4 and removal of trees and a generally low GHG emission the option of ecological restoration was not regarded as an immediate asset for climate change reduction but only in a time span of centuries.
Fertilization as part of a future commercial forestry option (option 3) was expected to reduce the greenhouse gas emissions for decades, as tree biomass increases. However, in a longer time scale over several rotations, intensive forest management would have a climate warming impact due to progressive loss of carbon from the peat due to maintenance of the ditches by new drainage activities (Juutinen et al. 2019, Juutinen et al. 2020).
The peat production land use scenarios 5–7 involving peat excavation were contributing to climate warming due to the release of large amounts of CO2 from drainage of the soil (Juutinen et al. 2020) and potentially also peat burning or the use of Sphagnum for horticulture.
Biodiversity
Of the seven land use scenarios described in the land use section three can be expected to be beneficial to biodiversity. Ecological restoration (option 4) had the most immediate effect on reestablishment of original species richness. Ecological restoration aims at restoring natural hydrology and removing trees and invasive species creating suitable conditions for mire species (going towards a more pristine-like and intact mire ecosystem) (Juutinen et al. 2020).
Leaving sites as they are (option 1) may achieve positive results for biodiversity, but it may take longer time sometimes much longer because the water table only gradually will rise and the trees stimulated by forestry was not considered removed in this option, which will have negative consequences for the water balance. On the other hand removal of the trees in the peatland would in many cases result in increased load of nutrients into the water. Hence, it appears that the “leaving sites as they are” may work for biodiversity under some conditions dependent on the initial degradation of the site, opportunities for self-restoration and load of nutrients.
Option 2 involving immediate wood harvested for bioenergy was expected to enhance biodiversity because the peatland will be left to re-establish itself after this one-instance intervention of removing trees but the actions is on the other hand expected to increase water pollution by release of nutrients as mentioned above (see also Tolvanen et al. 2020a). Again, this option will work under conditions with a low load of nutrients.
Option 3 involving intensive forestry is expected to decrease biodiversity and the richness of mire species significantly by continued fertilization and drainage (Kurttila et al. 2020). Finally, interventions involving peat extraction (options 5–7) are regarded as detrimental to biodiversity because the entire mire habitat will be destroyed. This is in this report considered the case even though the excavated area may at a later stage subsequently be rewetted because the original set of mire species have been lost.
Water quality impacts for each option were estimated for total nitrogen, total phosphorus and total organic carbon, which are contributing to water eutrophication and with a negative impact on the development towards a more original set of biological communities. Ecological restoration minimized nutrient impact in the peatland thus providing the best overall contribution to improve water quality of the seven options.

Fig. 6.4.3. Predicted number of red-listed mire plant species based on species distributions in the present state and under the six restoration scenarios (15% to 100% restored peatland area). Colors indicate the number of species for which the habitat is suitable according to the model (threshold 0.9 and 20 km dispersal limit). The total number of modelled species was 48. Source: Tolvanen et al. 2020b. As can be seen a 15% restoration area will not be as effective as larger restoration areas, which will facilitate dispersal more.
Tolvanen et al. (2020b) investigated by modelling habitat suitability of 48 mires species in six scenarios when 15%, 30%, 45%, 60%, 75% and 100% of the drained Finnish peatlands were restored (Fig. 6.4.3). Their main finding was that a large restoration area would expand the distribution of the mire species considerably more than a small area corresponding to a restored area of 15%. Thus, Tolvanen et al. (2020b) advocated for a landscape level approach to assess thresholds for planning for the potential biodiversity benefits.
In total 223 vascular plants and mosses (bryophytes) with peatlands as their primary habitats and 420 with peatlands as one of their habitats are threatened (red-listed) in Finland (Saarimaa et al. 2019 and references therein).
Evaluation of evidence
Climate
Flux studies have been conducted on forestry-drained peatlands in a number of Finnish studies and with a large number of publications in per-reviewed international journals. Ojanen et al. (2010) based their findings on samples from 68 study sites over two years of those parts of Finland where drainage for forestry is economically viable. In another study Ojanen et al. (2018) conducted soil flux measurements eleven times over a year at 49 sites with different fertilization pressures. The results of these studies are referred to above.
Climate impact in the land use scenarios was estimated as a radiative forcing time series based on the IPCC mean life times and radiative efficacies of the greenhouse gases (Juutinen et al. 2020). To calculate radiative forcing, annual greenhouse gas emission and removal time series were calculated for each option for each forest stand. CO2, CH4 and N2O flux exchanges between soil and the atmosphere, changes in tree stand carbon storage and emissions from the burning of peat were included in the calculation (Juutinen et al. 2020 and references therein).
Emissions of potential peat production sites were calculated as the difference between the total greenhouse gas emissions (Mg CO2 equivalents, GWP100) for the peat production option and the current land-use option until the end of the peat production (LIFE 2018, Juutinen et al. 2019).
Biodiversity
The habitat suitability model predicting the occurrence of mire dependent plant species has been described in detail in per reviewed international journals (Saarimaa et al. 2019, Juutinen et al. 2020) as well as the model study by Tolvanen et al. (2020b) on species distribution recovery in different area restoration scenarios.
Overall, results are comprehensively documented in a number of peer-reviewed papers in international scientific journals (see reference list). A next step may be to implement and monitor practical implementation projects at a larger scale to test how they match the models and to gain further practical experiences.
Synergy
Priority to biodiversity should be given in areas with large values e.g. large numbers of threatened species or high potential for restoration taking into consideration also size of areas, opportunities for creating functional connectivity and location of other high biodiversity areas. Priority to mitigate climate change should take into consideration depth of peat layers and current emissions from sites.
For a number of nutrient poor degraded peatland areas leaving these with no further action may seem as an acceptable option for both climate and biodiversity because present emissions often are very low and with time these areas will increase their biodiversity values due to a natural increase in water table and rewilding. An initial felling of trees is expected to enhance biodiversity but at the expense of climate mitigation in the short term and with a potential negative effect on water quality (negative effect on biodiversity) unless carefully planned.
Synergy at the site level may sometimes be difficult because the studies show a trade-off in approximately 80% of the peatlands between biodiversity and climate during the next 100–200 years (Juutinen et al. 2019). According to these studies ecological restoration (option 4) will benefit biodiversity (is expensive) and does only benefit climate in a longer time perspective due to an expected immediate release of CH4 and CO2 emissions from burning the removed trees.
Generally, the studies showed the increased advantages for synergy through careful landscape planning providing for different management options and identifying areas with different suitability in relation to biodiversity and climate change mitigation. Thus, providing sometimes different priorities for different areas and thereby maximize synergy between biodiversity and mitigation of climate change at the regional landscape scale. Careful participatory planning e.g. as demonstrated by the use of the YODA tool can at the same time assist in providing acceptable solutions to municipalities and private landowners.
Acknowledgement
Many thanks to Professor, Anne Tolvanen, Natural Resources Institute, Oulu, Finland (Luke) for information and references and comments to an earlier draft and to Jari Ilmonen, Metsähallitus, Parks & Wildlife Finland for information on Finnish peatlands.
6.5. Natural regeneration of temperate deciduous forests, Norway
Introduction
Temperate deciduous forests with native broadleaved tree species are important for biodiversity as well as for climate change mitigation in Norway along with old-growth coniferous forests on rich soil and a rich ground vegetation (Framstad et al. 2011). Therefore, their restoration and conservation may constitute a Norwegian contribution to relevant international policy agreements within these areas. This case presents an opportunity to restore deciduous forest biodiversity and at the same time contribute to build up the ecosystem carbon stock. The case deals with a small fraction of the total Norwegian forest area only, however, it is an illustrative example of synergy between biodiversity and climate regulation under limited human intervention.
Vigorous temperate deciduous forest used to cover large areas of Europe, extending from Central Europe to southern Scandinavia (Olsen et al. 2020). It was mainly found in areas with fertile soils attractive to agriculture, which has caused large scale land conversion, and today temperate deciduous forests is one of the most deteriorated ecosystems in the Nordic countries and globally (Götmark 2013, Olsen et al. 2020). Of a total forested area in Norway estimated at about 122,000 km2 (Flugsrud et al. 2016) an area of about 1,500 km2 (Framstad et al. 2011), corresponding to 1.2%, was estimated to be temperate deciduous broad-leaved forests (> 30% native broadleaved tree species).
Areas previously covered with temperate deciduous forest are often capable of natural regeneration. Temperate deciduous tree species have already recolonized abandoned cultivated land during the last 40–80 years (Olsen et al. 2020) forming new forests, which now covers about 530 km2 in Norway and 960 km2 in Sweden, but the overall potential for regeneration may be significantly larger. However, the newly colonized areas are generally mixed deciduous and coniferous forests with a substantial element of mainly Norway spruce (Picea abies). Active restoration by removing spruce may benefit the regeneration of deciduous forest or may even be necessary for successful regeneration in the long term (Olsen et al. 2020).
Case
Temperate deciduous forests in Norway comprise native broadleaved tree species such as Elm (Ulmus glabra), Ash (Fraxinus excelsior), Oak (Quercus robur) and (Q. petraea), Hazel (Corylus avellana), Lime (Tilia cordata), Maple (Acer platanoides) and Beech (Fagus sylvatica). These forests are confined to warmer climatic conditions in the southern part and along the Atlantic coastline (Olsen et al. 2020) and do also occur in southern Sweden, while boreal coniferous forests dominate in both countries (Nordén et al. 2019).
Studies conducted by the Norwegian Institute for Nature Research (NINA) has targeted temperate deciduous forests in collaboration with Swedish research colleagues (Nordén et al. 2019, Olsen et al. 2020). Biodiversity and carbon stocks were examined in recently established deciduous woodlands on abandoned farmland at 13 sites in Norway and 13 sites in Sweden (Nordén et al. 2019). These studies describe the potential for ecosystem restoration of these temperate deciduous forests and the results for biodiversity and climate change mitigation as well as possible economic incentives.

Fig. 6.5.1. Recently established temperate deciduous woodland locations investigated in Norway and Sweden. Map of Europe (middle) showing the locations of the 26 sites in Southern Norway (left) and Sweden (right). Numbers refer to the specific localities in the project. Source: Nordén et al. (2019).
Data were collected on biodiversity, biomass and present management practices, as well as potential economic incentives for landowners for restoration activities. The recently developed deciduous woodlands are generally young and dense, and mixed with Norway spruce, which often spreads from plantations (Nordén et al. 2019, Olsen et al. 2020).
An important element in this case was to assess the effect on carbon stocks and biodiversity of active restoration through thinning, mainly by removal of spruce from the developing deciduous forests (Olsen et al. 2020). Moreover, the income generation from this activity was addressed (Nordén et al. 2019).
Land tenure and protection status
Abandoned agricultural areas with regeneration of deciduous forest trees is a mix of private, municipality and state-owned land. Thus, the 26 investigated localities comprised 12 private, 5 state, 8 municipal and 1 mixed private/state ownership. Private landowners were generally positive towards restoration of deciduous forests and for the public to get access to these forests (Olsen et al. 2020).
Thirteen of the 26 investigated locations or parts of these were covered by various conservation schemes including Landscape Protection Areas, Nature Reserves, National Parks, Plant and Wildlife Protection Areas, Woodland Key Habitats or Protection-worthy State Owned Forests. The remaining areas were not subject to any formal protection.
Current land use and management
Most of the native deciduous forests in Norway have been converted to agriculture, but abandoned areas are now recolonized by young forests. The future management of these developing forests remain open and they are presently unmanaged in general. This implies that decisions should be made about the future management of these areas.
Recolonized deciduous or mixed forest with an age of 40–80 years has been estimated to occupy about 1,490 km2 of abandoned agricultural land in southern Norway and Sweden (Nordén et al. 2019). It was also estimated that about 1,000 km2 of these areas was the most relevant in relation to active restoration, because deciduous trees make up less than 75%. However, the overall potential for regeneration of broadleaved forest may be significantly larger. Thus, land use abandonment, in a long-term perspective, is assessed to have the overall potential to support about 50,000 km2 of natural forest regeneration in Norway (Bryn et al. 2013). Only the southern regions, and mainly coastal areas, are climatically suitable for temperate deciduous forest as shown in Fig. 6.5.2.

Fig. 6.5.2. Vegetation map of Norway modified after Moen (1987). The green areas are the main zones suitable for deciduous forest growth in Norway. Source: Panitz et al. 2016. Climate of the Past.
A further indication of these areas are given by the present distribution of deciduous species of Ash and Oak, also where they do not make up actual woodlands (Fig. 6.5.4, 6.5.5).

Fig. 6.5.3. Detailed distribution of Ash (Fraxinus excelsior) in Southern Norway indicated by black dots. Records of Ash trees are seen in three climatic zones i.e. Nemoral (red), Boreonemoral (yellow) and Sørboreal (orange), which are the zones of potential for temperate deciduous forest restoration. Scattered records can also be found in Mellemboreal. Source. TRANSFOREST project, NINA.
The far majority of the forest in Norway today, is managed as production forest with commercial thinning and harvest by clear-cutting corresponding to about 91% of the area of production forest (Bartlett et al. 2020). The effect of logging might be considerable, as transporting biomass from the forest means removing a large stock of carbon (and nutrients) from the ecosystem, and hindering transport of carbon to the soil by trees and other plants and their associated biological communities, including their fungal (mycorrhizal) partners (Bartlett et al. 2020). Commercial thinning is done 1–2 times in young to middle-aged forests, and final felling is usually done when the age of the dominating trees is 60–120 years leaving about 2.5% of the productive forests older than 160 years (Tomter & Dalen 2018, Bartlett et al. 2020). Thus, this may lead to a substantial decrease in the input of carbon into the soil (Liski et al. 1998).

Fig. 6.5.4. The distribution of records of Oak Quercus robur and Q. petraea (green, left) and Fraxinus excelsior (red, right) in Norway from Artsdatabanken og GBIF-Norge: https://bit.ly/38nmaa7 . The maps taken together provide an indication of the potential distribution of deciduous forest “edelløvskog” in Norway.
Stakeholders
The current landowners are the primary target group for the future management of these reforested areas (Nordén et al. 2019), in collaboration with local and national authorities on agriculture, environment and forestry. Other interest groups include outdoor recreation organizations, environmental organizations, scientific researchers and NGOs (Rush in lit.).
The studies (Nordén et al. 2019, Olsen et al. 2020) indicated that landowners did not benefit economically from the wood biomass harvest, that were carried out to support the restoration of deciduous forest. Nor did they believe that these interventions were economically attractive in a future perspective. Obviously, the income depends on the amount of biomass taken out per area. Furthermore, the area manipulated at each site in the study was very small (Nordén et al. 2019). Hence, the development of alternative incentives for landowners are generally needed (Olsen et al. 2020).
Climate
The carbon stock in the biomass of living trees in the 26 regenerated deciduous forests examined were estimated at between 270 and 1,180 ton CO2-eq per ha before thinning (mainly by removal of Norway spruce) (Olsen et al. 2020). The variation among locations mainly depends on the fertility of the soil and the stage of succession (age of the recolonizing woodland). The density of trees, for example, was negatively correlated with standing biomass because younger woodlands had higher density and smaller trees, while older woodlands had lower densities, but larger trees, and often with a larger proportion of spruce (Olsen et al. 2020).
In order to estimate the future biomass development, a so-called T model (Olsen et al. 2020) was applied. The estimated accumulation of biomass in a 100 years perspective corresponded to 1.89 ton and 1.51 CO2-eq per ha per year in thinned and un-thinned forest stands, respectively (Fig. 6.5.5). The model estimates also included the development of soil organic carbon over 100 years (Fig. 6.5.5). The carbon accumulation in the soil was roughly half of that in the biomass, but almost identical for forests with or without active restoration (thinning by removal of Norway spruce). The accumulation in the soil was almost linear throughout the modelling period, in contrast to the biomass. However, the model predictions should be taken as indications only due to a high uncertainty, especially on the estimates on soil carbon (Ťupek et al. 2019).

Fig. 6.5.5. Modelled development of biomass in developing temperate deciduous forests over 100 years: In the living tree biomass (A) and the soil (B). Blue line is the average accumulation for forests with removal of exotic tree species and dotted orange line is average for untouched forests. The blue and orange bands show the corresponding variation in the data. On X-axis years and on Y-axis change in living biomass (A) and carbon in soil (B) in tons of dry matter per ha. Source: Olsen et al. (2020).
In addition to the specific studies on deciduous forest above, The Norwegian Institute for Nature Research (NINA) has published a number of desk top studies, which thoroughly assess the importance of Norwegian forests for carbon uptake and storage, and the significance for climate change mitigation (Framstad et al. 2011, Rusch 2012, Bartlett et al. 2020). These studies are based on Norwegian data from e.g. the national forest inventory and environmental monitoring as well as local scientific studies and the international scientific literature in general. Without going further into these assessments, some of the important finding in this context were as follows:
The largest carbon stocks per unit area are found in old forests, especially old spruce and mature deciduous broad-leaved forests. The annual CO2 uptake per unit area is largest in the more productive forests, such as rich deciduous and coniferous forests and productive mature deciduous forest. The term “rich forest” follows the classifications of the Norwegian Directorate for Nature Management and is based mainly on nutrient and ground vegetation criteria.
Forests consisting of more diverse set of tree species tend to have higher carbon stocks than forests that consist of only one or a few tree species and old stands had higher C stocks than young ones.
Several studies suggest that old‐growth forest stands and soils still take up more carbon than they emit, and they may therefore act as carbon sinks for hundreds or even thousands of years.
Overall, these findings suggest that restoration of natural, temperate deciduous with a natural mixture of native tree species, will provide long term climate benefits in terms of uptake of CO2 and increasing carbon stocks.
As already described, active restoration by removal of coniferous trees may further increase the climate benefits. However, actual commercial logging in the long term will limit the accumulation of carbon in tree biomass and deadwood and most likely also in the soil, as is also discussed by Framstad et al. (2011). On the other hand, harvested wood products contribute to climate mitigation by substitution of fossil fuels and GHG-intensive materials like concrete and steel. The long-term significance of this is uncertain, however, and depends strongly of the development in other sectors.
The general trade-off between the substitution benefits from wood production and the carbon storage of unmanaged forests is currently highly debated and beyond the scope of this case study. However, it is discussed in a Norwegian setting by Bartlett et al. (2020) who conclude that “Overall, increased use of wood products and wood-based fuels creates a carbon debt in the forest that is not compensated for if the life time of the wood products or fuels is shorter than the time it takes for the new forest to recreate its carbon stock.”
Biodiversity
Old deciduous broad-leaved forests have been classified as one of the most valuable forest types in Norway in terms of biodiversity conservation in Norway (Framstad et al. 2011, Fig. 6.5.6). As stated in the introduction, the total area of this forest type in Norway is very small and few studies in natural regeneration of deciduous forest on abandoned land have been carried out (Fig. 6.5.7). However, many nationally threatened species have been found in such areas and several of them are abundant (Norden et al. 2015). In an analysis of 690 deciduous trees at 65 localities, a total of 34 nationally red-listed lichens and 14 red-listed wood- or bark-living fungi on were recorded on the living trees (Nordén et al. 2015). Elm was significantly richer in red-listed species than Ash, and larger trees had more red-listed species than smaller.
Preliminary investigations summarized in Olsen et al. (2020) indicate that the recolonizing deciduous woodlands already contain rather diverse communities of plants, lichens, insects and dead wood fungi. A total of 920 species of insects have been recorded in the developing deciduous forests. The results also showed that thinning and the removal of Norway spruce while retaining tree species with conservation values enhanced the conditions for various insect groups including beetles, butterflies and hoverflies (see Fig. 6.5.8). However, the diversity is expected to increase further in the long term. Thus, low abundance of species characteristic of temperate deciduous forests and few nationally threatened species were found compared to (comparable) old growth forests, which is not surprising taking the young age of these developing forests into account.
To improve biodiversity, an initial and perhaps sometimes repeated intervention of selective cutting of specifically Norway spruce would be beneficial. This may even necessary in order to restore the deciduous forest and preserve it in the long term (Olsen et al. 2020),

Fig. 6.5.6. Temperate deciduous forests (here in Norway) once covered the southern parts of the Nordic region on rich soil and the entire Europe. They include some of the most threatened nature types today because the fertile soil has been widely used for agriculture. Photo: Kristin Thorsrud Teien.

Fig. 6.5.7. Young mixed forests on abandoned agricultural land provide an opportunity for ecological restoration of the formerly more widespread deciduous forests ecosystems. Photo: Siri Lie Olsen.
Fig. 6.5.8. Average number of individuals (Y-axis) of insects (beetles, butterflies and hoverflies) in managed and unmanaged study plots in temperate deciduous forests before (2016) and after thinning (2019) X-axis. The increase in individuals is significantly higher in the managed forests (grey bars) with removal of mainly spruce trees compared to controls without intervention (red bars). Source: Olsen et al. (2020).
Evaluation of evidence
The results are summarized in “Temahefte 77” (Olsen et al. 2020) published by the Norwegian Institute for Nature Research (NINA) and supported by several articles in international peer reviewed journals (see reference list).
Synergy
Sparing abandoned land for regrowth of temperate deciduous forests in Norway and Sweden provides an opportunity for restoring important biodiversity. At the same time, a long-term increase of carbon uptake and storage in both wood biomass and soil is expected, which will constitute a positive contribution to climate change mitigation.
Active restoration of deciduous forests by removal of the Norway spruce is shown to benefit biodiversity. It may also increase the uptake and storage of carbon and thus the climate benefit. The climate benefit will further depend on the use of the harvested wood products, as it will increase with the proportion of the wood used for construction and long-lived products rather than direct energy uses.
The potential for temperate deciduous forest to grow in Norway (and Sweden) is expected to increase due to climate warming. Therefore, a strategy to adapt to these climatic changes by promoting this forest type seems to be a long-term investment in biodiversity and both climate adaptation and mitigation.
Acknowledgement
Our sincere thanks go to Lajla Tunaal White, The Norwegian Ministry of Climate and Environment for input to the report and facilitating contact to researchers in NINA and here to Björn Nordén, Jenni Nordén, and Graciela Rusch for many good comments to an earlier draft as well as assistance with information on Norwegian forests as well as to Zander Venter for production of the vegetation map.
6.6. Restoration of an almost extinct forest ecosystem, Iceland
Introduction
Iceland is a young country on a geological scale. Its most ancient rock is less than 20 million years old (Arnalds 2015) and throughout the last 2–3 million years (Quarternary), ice sheets covered most of Iceland during the major glacial periods. Situated in the North Atlantic about 290 km from Greenland and 970 km from Norway, Iceland is isolated from other landmasses. This makes it difficult for plants and animals to disperse to Iceland, and Holocene colonization (i.e. since last glacial period) is very slow. Therefore, the biodiversity is not very high and only few endemic species are found (Anonymous 2001)
The only native tree in Iceland that forms woodlands is downy birch (Betula pubescens). Nevertheless, it is estimated that birch woodlands and shrub covered more than 25% or up to 40% of the land area at the time of settlement about 1100 year ago (Sigurðsson 1977, Wöll 2008). Over the following centuries, extensive deforestation took place due to clearing for pastures and hay fields, grazing, and exploitation for firewood, charcoal and timber. Afterwards, the deforestation continued at lower rates until about 1900, and eventually almost all native forest was lost. Today, birch woodlands cover around 1,500 km2, or 1.5% of the land area. Around 70% of these are scrublands lower than 2 m, and only 1% are forest with trees higher than 5 m (Snorrason et al. 2016).
The deforestation and subsequent land use, mainly sheep grazing, has been a major factor leading to extensive ecosystem degradation in Iceland, with widespread wind and water driven soil erosion and desertification. These severe impacts of deforestation is due to the properties of the domination volcanic soils of Iceland (andosols) in combination with natural processes like volcanic activity, ash deposition and flooding (Arnalds 2015). Soil erosion has long been recognized as a major environmental problem of Iceland. Thus, the Icelandic Soil Conservation Service was established already in 1907, mainly to battle advancing sand, which was destroying farms in Southern Iceland (Arnalds 2015). During the recent decades there is an increasing awareness of the negative impact of the historical deforestation and ecosystem degradation on biodiversity and natural carbon stocks (e.g. Anonymous 2001, Aradóttir et al. 2013)
This case study describes the status of native birch forest in Iceland and discuss the significance and perspectives of these woodlands and their restoration for biodiversity conservation and for climate change mitigation through uptake and storage of CO2 (sequestration).
Area
Today, birch woodland and scrub in Iceland occupy about 1,500 km2. Most of this is found in small scattered occurrences distributed across the country at elevations below 300 m (Fig. 6.6.1, top). Wöll (2008) estimates the area climatically available for growth in modern Iceland to be more than 40,000 km2 or 40% of Iceland’s land area (103,000 km2), which can be regarded as the potential area available for ecological restoration of birch woodland. It may also represent a fair estimate of the distribution before the time of settlement (Karlsdottir 2014), although a commonly used estimate is 28,000 km2 (Sigurðsson 1977). See Fig. 6.6.1 bottom. Examples of high and low stature birch woodland and scrubland in Iceland are shown on Fig. 6.6.2.

Fig. 6.6.1. Top: Present distribution of natural (or planted) birch woodland in Iceland according to the latest national inventory in 2010–2014 (from Snorrason et al. 2016). Bottom: Estimated distribution of birch woodland at the age of human settlement about 1100 years ago (dark green) (Iceland Institute of Natural History 2001, figure from Trbojević 2016).

Fig. 6.6.2. Top left: High stature of birch woodland (> 5 m height at maturity) in Vaglaskógur forest in North Iceland (Photo: Arnór Snorrason). Top right: The highest natural birch tree measured in Iceland was 14.2 m in autumn 2016. It grows in Vaglaskógur forest in North Iceland (Photo: Rúnar Ísleifsson). Bottom: Low stature of birch woodland (scrubland, < 2 m height at maturity) in Reykjanes peninsula in Southwest Iceland. (Photo: Arnór Snorrason).
Land tenure and protection status
Approximately 70% of forests and woodlands in Iceland are private, with the remainder owned by the national government or local municipalities (ACIA 2005). According to Aradottir & Eysteinsson (2005), state owned land includes about 15% of Icelandic birch woodlands as well as adjacent areas suitable for their restoration.
Harvesting of wood has been limited and regulated for more than a century, and since 2015 native birch woodlands and their remnants has enjoyed protection pursuant to the national Nature Protection Act. Through the last decades, grazing of livestock, sheep in particular, has been the main threat to the birch woods and the principal obstacle to their expansion. Despite the declining grazing pressure today, free roaming sheep still affect the birch woodlands, especially because they prevent the regeneration of birch in new areas. A former threat from recreation development projects is now of minor significance (Óskarsson, Oddsdóttir and Svarvarsdóttir pers. com.).
Current land use, human activities and stakeholders
Even though sheep farming is declining, summer grazing in natural birch woodlands and in areas suitable for restoration is still common. Recreational activities are also common in many areas, ranging from wilderness hiking to the development of tourist accommodation facilities.
The most important stakeholders are the landowners, most of which are private farmers. Local and national authorities are stakeholder both as landowners and as authorities regulating forestry and the use of forest in general, including nature protection. Other important stakeholders include local and foreign tourists and the public in general. In Iceland, there is a general right to cross uncultivated private property without seeking any special permission, although landowners can limit routes with signs other marks. State-owned land such as conservation areas and forestry areas are generally open to everyone.
The most important actors in Icelandic afforestation are government-funded afforestation projects and forestry societies (NGO’s) (Aradottir & Eysteinsson 2005).
Climate change regulation
The main relevance of natural birch forests in Iceland in relation to climate change mitigation is the carbon stored in biomass and soils, which is generally larger than on barren land or treeless rangeland (Snorrason et al. 2002, Bárcena et al. 2014). As the extent of birch woodlands today is greatly diminished compared to the original distribution, there is a significant potential for future carbon sequestration if woodlands are restored (Fig. 6.6.1). In accordance with this, afforestation and revegetation initiatives has become increasingly motivated climate change mitigation (Aradóttir et al. 2013) and afforestation is part Iceland’s climate change strategy (Ministry for the Environment 2007).
Snorrason et al. (2019) estimated the total above-ground woody biomass in the natural birch forests in Iceland at 1503 kt in 1987–1988 and 1455 kt in 2005–2011, for the woodland already existing in 1987–1988 (1380 km2). Based on this, they concluded that the biomass did not change significantly between these two national inventories in older existing natural forests. However, in Iceland, the native birch woodland is in a continuous cycle of regeneration, growth and tree mortality (Jónsson 2004) and both biomass increments and turnover rates are low compared to the standing stock. Thus, in the 20-year period, significant biomass changes were not to be expected (Snorrason et al. 2019). However, an increment of 37 kt woody biomass from natural birch expansion to formerly treeless areas, was estimated for the same period. This was based on another study showing that 129 km2 of natural birch woodlands colonized an area of previously open land from 1989 to 2012 (Snorrason et al. 2016), corresponding to a mean annual rate of 563 ha.
The above results suggest that natural regeneration of birch woodlands has not yet contributed significantly to climate change mitigation. However, the biomass estimates can be used for simple scenarios of the future potential. For example, restoration of birch woodland to half of its original extent would take up at least 14,000 km2 of land. If we assume that the aboveground woody biomass in these areas reach levels similar to the present woodland (approx. 1.1 kt per km2) this would result in an additional biomass of about 15,400 kt in total, corresponding roughly to a carbon sequestration of 28,000 kt CO2-eq (CO2 equivalents). For comparison, Iceland’s total emissions in 2018 was 4,900 kt CO2-eq for all sectors without LULUCF (Land Use, Land Use Change and Forest) and 13,900 CO2-eq with LULUCF (Keller et al. 2020). The latter is so much higher due to large-scale historical wetland draining for agriculture in Iceland.
The above estimates suggest, that restoration of birch woodlands to half its original distribution would entail a carbon sequestration in the woody biomass corresponding to Iceland’s present emission in two years. However, an additional – and larger – carbon sequestration can be expected in the soil, amounting to maybe 1,900 kt per year, or 14% of Iceland’s annual emissions. This estimate assumes an average sequestration in the soil of 134 g CO2-eq per m2 per year following afforestation, which was applied in latest National Inventory Report on the emission of greenhouse gases (Keller 2020). This estimate was based on several studies showing a substantial carbon sequestration in the soil following afforestation in Iceland, both in unvegetated areas and on grasslands or heathlands (References in Keller 2000, but see also Owona 2019).
Although these are very crude estimates, they indicate that restoration of birch woodland could make up a significant contribution to climate mitigation in Iceland. On the other hand, with the recent natural woodland expansion rate (563 ha per year, 1989–2012) it would in theory take more than 2000 years to fulfill the scenario above. This means that the natural expansion must be boosted by active restoration measures in order to contribute significantly to meet the Paris Agreement requirements. Another challenge to large scale restoration of natural birch woodlands may be competing existing land uses like livestock grazing, or other future land uses such as forestry based on imported coniferous tree species. While the latter may contribute even more to climate change mitigation, it will contribute less to biodiversity conservation of native ecosystems.
Biodiversity
Sub-Arctic birch woodlands and shrub are the only indigenous woodland types in Iceland. Examples of these woodlands are shown in Fig. 6.6.3. Similar birch woods form the tree line in South Greenland, Scandinavia and the Kola Peninsula of North Russia (Jónsson 2001). Downy birch (Betula pubescens) is almost the only tree species in most natural woodlands and scrub in Iceland. Occasionally, Rowan (Sorbus aquiparia), and even more rarely Aspen (Populus tremula), are found among the birches. Tea-leaved willow (Salix phylicifolia), Woolly willow (Salix lanata) and prostrate Juniper (Juniperus communis) are also common shrubs in birch woodlands (Jónsson 2001).
As described above, birch woodlands and scrub used to be widely distributed in Iceland and may have been the most common ecosystem and natural habitat in Iceland’s lowlands, covering 28–40% of land (Wöll 2008). Thus, the historic removal of more than 95% of this habitat has substantially affected biodiversity at the ecosystem level. While the deforested land has suffered degradation, in some cases even desertification, it is assessed that the remaining birch woodland retain most of their original biodiversity (Jónsson 2001). The birch woods are home to a number of nationally red listed species and the native birch woodlands and scrub are key areas of terrestrial biodiversity in Iceland (Jónsson 2001).

Fig. 6.6.3. Left: Icelandic woodland of medium height (2–5 m at maturity). Photo: Arnór Snorrason. Right: Þórsmörk after volcanic ash deposits covered birch woodlands in the Eyjafjallajökull eruption in 2010. The forest survived and healed in few weeks.
Through more than a decade, initiatives have been taken to restore woodlands and forest in Iceland, mainly to prevent soil erosion (Aradóttir et al. 2013). When first established, the birch woodlands have a remarkable ability to survive impacts from volcanic activity (Fig. 6.6.3, right). While soil conservation is still a major goal of afforestation, restoration of birch woodlands is now increasingly motivated also by nature conservation (e.g. Aradottir et al. 2013), and ecological restoration is part of the Iceland’s Biodiversity Action Plan (Anonymous 2014).
Several studies have addressed ecosystem function and biodiversity of Icelandic birch woodlands and the perspectives in relation to of restoration. Generally, birch is able to colonize new land and establish woodland within few decades if conditions are suitable; i.e. most notably if livestock grazing is excluded or reduced to low levels. This is documented for e.g. natural succession on an outwash plain in front of a retreating glacier (Marteinsdóttir et al. 2007) and after exclusion of sheep grazing in heathland (Behrend 2019).
The ICEWOOD project studied afforestation in two areas in eastern and western Iceland, respectively. Biodiversity and carbon cycling in larch, pine, and spruce plantations were compared with remnants of the native birch woodlands and open heathland (Elmarsdottir et al. 2007, 2011, Halldorsson et al. 2007 and references therein). The studies indicate that species richness increase along a gradient from heathland to young forest, while it seems to fall in older forest and woodland stands (Elmarsdottir et al. 2011). Apparently, there is no systematical difference in species richness between natural birch woodland and the other habitats. However, the dominance pattern among species as well as taxonomical and functional species groups changes with forest type and stage. The diversity of fungi and soil collembolan (springtails) increase with stand age, while the diversity of vascular plants, mosses and lichens seem to decrease. The lowest plant richness is found in coniferous plantations in the thicket stage before the first thinning (Elmarsdottir & Magnusson 2007, Elmarsdottir et al. 2011).
Generally, it seems that no or only very few specialist forest species are found in Iceland. Another conclusion is that both species richness and species composition are generally governed by the light penetration to the ground, more than the dominant tree species (Elmarsdottir & Magnusson 2007, Elmarsdottir et al. 2011).
The natural subarctic birch woodlands are relatively open even at maturity, allowing for more light penetration than mature conifer plantations, and thus a higher species richness, and more natural species composition and dominance patterns. In terms of area and number of trees planted, afforestation in Iceland presently relies mainly on plantations with imported conifer species, such as larch, pine and spruce, with wood production as an important management goal (e.g. Aradottir & Eysteinsson 2005). Thus, there is a potential conflict between wood production and biodiversity (Elmarsdottir & Magnusson 2007). This conflict may be partly handled through active management for biodiversity in the plantations. However, in order to seriously address biodiversity conservation at both ecosystem level and species level, dedicated efforts for restoring natural birch woodlands are essential.
In relation to restoration, the Hekluskógar afforestation project in Iceland is highly relevant. Its aim is to restore maybe 700–800 km2 of natural birch forest on heavily degraded land in the vicinity of the volcano Mt. Hekla (Aradóttir 2007, Óskarsson 2009, Óskarsson et al. 2011). The project is coordinated by the state agencies Icelandic Forest Service and Soil Conservation Service of Iceland, while the practical activities in the field are mainly handled by landowners and volunteers. Large-scale restoration of the woodland in Iceland needs to work with the natural processes, enhancing regeneration through stimulating seed source and safe sites (Aradottir & Halldórsson 2018). The restoration actions of the Hekluskógar project include revegetation with non-wood plant species for site amelioration for birch colonization as well as planting of birch in small stands, relying on natural regeneration of birch over the remaining area.
With its large ambitions, the Hekluskógar project must be regarded as a flagship project at a European scale, and very much in line with the Convention of Biological Diversity (CBD) and international recommendations from both IPBES and IPCC. Nevertheless, even this large-scale project will restore “only” 2–3% of the native birch woodland in Iceland. For comparison, the Aichi goal 15 of the CBD for 2020 was restoration of at least 15% of degraded ecosystems.
Evaluation of evidence
This case study is based substantial evidence, published mainly in peer reviewed scientific papers and reports from Islandic government agencies; and several leading Icelandic experts on the main issues have been consulted. The historical deforestation in Iceland is very well documented, as are the present distribution and ecology of the natural birch woodlands and ongoing restoration initiatives. It is also well established that restoration of birch woodland can contribute significantly to both biodiversity conservation and climate change mitigation. However, the presented quantitative estimates of the potential climate benefits are uncertain, as are the long-term consequences for biodiversity at the species level.
Synergy
Restoration of birch scrub and woodlands in Iceland through planting or natural and assisted spontaneous regrowth, will underpin both biodiversity conservation and climate change mitigation.
- Due to the historical almost total deforestation at the national scale, restoration of natural birch woodland is essential for the conservation of native biodiversity in Iceland at the ecosystem level. The importance at the species level is less clear as only few species, if any, depends on birch woodland as a habitat for their future survival.
- Birch woodland and scrub in Iceland store larger carbon pools in the biomass, and often even larger in the soil, than do barren land and treeless areas. On the other hand, especially the biomass of birch woodlands and scrub is low compared to Nordic and global forests in general. Thus, restoration of birch woodlands has a moderate but significant potential for carbon sequestration.
Acknowledgement
Several Icelandic experts have contributed through initial discussions of the case study and with important information and references, and valuable comments on the manuscript. For that we thank Hreinn Óskarsson, Edda S. Oddsdóttir and Brynja Hrafnkelsdóttir at the Icelandic Forest Service, Bjarni D. Sigurdsson at the Agricultural University of Iceland and Kristín Svavarsdóttir, Sunna Áskelsdóttir and Jóhann Þórsson at the Soil Conservation Service of Iceland.
6.7. Doubling the area of forest set aside biodiversity for conservation, Denmark
Introduction
In 2016, the Danish Government and Parliament agreed to set aside 13,000 ha (130 km2) of state forest for biodiversity conservation. Despite this modest area, it is the largest effort of the government to date to conserve forest biodiversity, and it will more than double the area of set aside forest in Denmark. In the political agreement it was stated that the areas should be set aside on the basis of the latest knowledge and under consultation of the relevant scientific research groups, and the effort should target the areas with the largest biodiversity potential.
In this case study we describe the area prioritization process leading to the biological research-based recommendations with focus on the stepwise multi-criteria approach, which was based on different types of biological data (Petersen et al. 2017). We also discuss the scope and limitations of the political agreement and its implementation in the context of the effort needed at the national scale to safeguard Danish forest biodiversity in the long term. Finally, we present estimates on the future carbon storage potential and climate mitigation effects of setting aside the forest.
Area
The Danish state owns about 110,000 ha of forest or about 18% of the total Danish forest area of about 630,000 ha, while the rest is mainly privately owned. The state forests are distributed across the country, but in an uneven pattern (Fig. 6.7.1). The areas along the west coast of the mainland, Jutland, are almost entirely coniferous plantations on nutrient-poor sandy soils, while the remaining areas are mixtures of broadleaved and coniferous stands on richer soils.

Fig. 6.7.1. Danish forest areas as distributed on state forests (purple) and other land owners (green).
Land tenure and protection status
Danish state forests are managed by the Nature Agency under the Ministry of Environment and Food. The Nature Agency is responsible for all activities, including commercial timber production, nature conservation initiatives and outdoor recreation.
Most forests in Denmark, including the state forests are protected to secure timber production under the Forest Act. This means that forests cannot be converted to other land uses and that owners must ensure planting or natural regrowth after logging. Apart from non-forest habitats like meadows and fens, Danish forests are not subject to any general nature protection regulations. However, some areas, are protected as EU Natura 2000 habitats, including 9% of the state forests. Most of these areas, however, are still subject to logging. Until the designations described in this case-study, the forest area formally set aside for nature conservation in Denmark amounted to only 9,000 ha of state forest and 12,000 ha in total. With a few exceptions these designations were distributed across many, often very small, areas in larger forests.
Current land use, human activities and stakeholders
Traditionally, the Danish state forests has been managed mainly for commercial wood production. However, since 1989, the importance of other purposes such as nature conservation and outdoor recreation, especially in state forests, has been clearly stated in the Danish Forest Act. Since 1995, a close-to-nature-forestry approach has been formally adopted in all state forests, and today all state forest are certified according to the two most common schemes, FSC and PEFC. However, timber production is still a major management goal in most forests. Until 2018, only 9,000 ha were formally set aside for biodiversity and nature conservation.
As a stakeholder, the Nature Agency has a particular dual role, because it is responsible for both timber production and nature conservation. The Agency’s budget thus depends to a certain extent on the income from timber production.
State forests are very important sites for recreational activities in large parts of the country. Public access to all areas at all hours of the day are secured by law. The Nature Agency establish and maintain facilities for many different outdoor activities, and state forests host numerous larger events. Thus, all kinds of outdoor user groups are very important stakeholders. Danish sawmills and woodworking industries are also important stakeholders, as they benefit from a stable local supply of especially certified wood from the state forests.
Biodiversity
It is estimated that almost two thirds of Denmark’s maybe 40,000 species are found in forests or related habitats, and that one third are confined to such habitats. Furthermore, the highest share of nationally threatened species are found among these obligate forest species. Despite of the modest area of forest in Denmark – 630,000 ha, or 15% of the land – the main threat to forest species are not the lack of forests as such, but rather the lack of various habitats in the forest due to the intensive forest management in most areas. No study specifically compares the biodiversity of state owned and private forests. There is no doubt that state forests are very important to the biodiversity in a few regions, but private forest are most likely even more important at the national scale. Not only do private forests make up 75% of the total forest area, in several regions, almost all forest are private (Fig. 6.7.1) and some of the most important known natural values are found in private forests.
The main aim here is to describe the selection and prioritization approach, which led to the research-based biological recommendations that were requested by the Ministry of Environment and Food in accordance with the political agreement to set aside state forest (Petersen et al. 2017).
The recommendations aimed at the greatest possible effect on the biodiversity within the framework and limitations of the political agreement through a database-based analytical approach. The process that led to the recommendations was divided into the following main steps:
- Screening of all state forest for areas of potential importance to biodiversity.
- Delineation of specific forest areas to be included in a total list of sites to be considered.
- Selection of the most important areas from the total list, in order to fulfill the requested total area quotas for broadleaved and coniferous forest, respectively.
- Prioritization of these proposed areas into three levels of importance.
Throughout the process, the areas were selected considering two general objectives:
- To ensure the designation of the best areas in terms of existing biodiversity and future potential for biodiversity conservation.
- To ensure a broad coverage of biodiversity in the state forests based on the principle of complementarity and geographical spreading.
All important information available were considered. The main data set included the following:
- National distribution of forest dwelling species. Presence and absence of 786 species in 633 10×10-km grid cells.
- High nature value (HNV) indicator. A high-resolution national index based on known records and distribution of red-listed species as well as landscape structural parameters, known occurrences of natural and semi-natural habitats and current land use.
- “2020-goal-species”. All Danish records (1991–2015) of 1236 species relevant to the political 2020-goals for biodiversity. This includes globally and nationally threatened species and species listed in the EU Habitats and Birds directives.
- Forest of particular natural value. Occurrences of forest of particular natural value in the sense of Section 25 in the Danish Forest Act, mapped on the basis of mainly structural forest features.
- EU forest habitats. Mapped occurrences of forest habitat types listed in the EU Habitats Directive.
- State forest management maps. Registration at the stand level of tree species and age, and areas already set aside for biodiversity conservation.
In addition to the data above, the process benefitted from the substantial local knowledge among the scientists involved, about the biodiversity and natural values in the state forests.
Spatial prioritization analyses on the principle of complementarity were given a high weight, particularly at the national scale, in order to ensure the broadest possible coverage of the biodiversity within the total area available. The HNV indicator was used to screen for areas of particular high value at the national level and for local prioritization and delineation. The number of threatened or unique species in the 10×10-km data were used mainly for local prioritization and delineation, as were the remaining data sets.
The political agreement aimed at the designation 10,000 ha of broadleaved forest and 3,500 ha of coniferous plantations. However, in order to allow for flexibility in the final designation by the Nature Agency, recommendations of about 17,000 ha of broadleaved forest and 6,000 ha of conifer plantations were requested. Furthermore, the designations should focus on entire forests or parts of larger forests, while previous designations had focused on small areas assessed to be of particular value to biodiversity.
The final recommendations (Petersen et al. 2017) included 42 mainly broadleaved forests and six coniferous plantations. Within the two categories, the areas were given priority 1, 2 or 3 according to their assessed potential for biodiversity conservation. Priority 1 represented very important forests that would be essential to fulfill the stated purpose of setting aside state forest. Priority 3 were forests, which could be designated if it was assessed – based on factors other than biodiversity – that there was a need to include areas other than 1st and 2nd priority areas. All in all, there was a good agreement among the areas between the different measures and indicators of biodiversity included in the analyses, indicating the robustness of the recommendations.
The Danish Nature Agency was responsible for the final selection and delineation of the future reserves (The Danish Nature Agency 2018). In addition to the research-based biological recommendations, this selection phase included research-based analyses of forest structures and economy as well as supplementary biological analyses and considerations, cultural history and associated legislation as well as access conditions and outdoor recreation opportunities for the public. The final designation amounted to a total of 13,800 ha distributed across 41 mainly broadleaved forest areas and four coniferous plantations (Fig. 6.7.2).
A subsequent evaluation (Petersen et al. 2018) showed that 80% of the total area of the final designations was located within 1st or 2nd priority locations of the recommendations, although with a different local delineation in several cases. Another 14% was within the 3rd priorities and 6% was completely outside the recommendations. All in all, this must be considered a reasonably good agreement with the recommendations, given that other aspects were considered. Most remarkable are the reserve in Gribskov in Northern Zealand including more than 3,000 ha of mainly broadleaved forest and substantial occurrences of wetlands. It has the potential of becoming one of the most outstanding temperate forest reserves in Europe. One significant issue, however, is that with a few exceptions the designated areas are smaller than recommended. This may reduce the efficiency of the initiative, as size of the individual area is critical to facilitate several important biological and ecological processes. It should also be noted that the inclusion of more than 3,000 ha of coniferous plantations in the set asides must be regarded as a political decision. Considering the distribution of threatened forest biodiversity in Denmark, the area had been better spent on more broadleaved forest.
Future management and the significance to biodiversity
Detailed management plans for most of the designated areas has not yet been established. At the general level, it is planned to stop commercial logging in the long term with the overall purpose of restoring more natural forest ecosystem. In many areas, some initial cutting will be carried out in order to increase the structural heterogeneity of uniform stands, and to create light gaps and clearings. Furthermore, exotic coniferous tree species will generally be removed and the widespread drainage by ditches will cease wherever possible. The use of fertilizers and pesticides had largely ceased in state forests even before the present initiatives. In the long term, most areas will be left without further intervention apart from actions aimed to restore natural grazing in selected areas either by introducing livestock or fencing naturally occurring deer. As grazing has been actively suppressed in Danish forests for more than 200 years, such initiatives are important to restore the full array of natural habitats. Finally, reduced logging will be allowed in some areas in order to preserve or promote certain species or habitats of particular importance to conservation.

Fig. 6.7.2. Final official designation of forest reserves in Danish state forests in 2018. Colours refer to the priority of the areas in the scientific biological recommendations. Numbers refer to the area (ha) set aside at each location. Based on The Danish Nature Agency 2018).
It is a challenging task to quantitatively assess the specific conservation effect of the planned forest reserves in terms of e.g. species diversity, distribution or probability of survival. This would require models that have not yet been developed for Denmark. However, at a more general level, there is every indication that the initiative will contribute significantly to the conservation of Danish biodiversity in the long term. First of all, this initiative alone will approximately double the area of forest set aside for biodiversity conservation in Denmark, from around 12,000 ha (2016) to approximately 25,000 ha, when fully implemented. The very fact that data-based biological considerations were given a high weight in the designation process, should contribute further to a positive outcome. Finally, it is well documented that European broadleaved temperate forest can host a very high biodiversity if allowed to develop old growth characteristics. One prominent Danish example is Suserup Skov located in central Zealand. Suserup Skov represents one of the best examples of semi-natural beech dominated forest in Northern Europe. The forest cover dates back to before 4200 BC; management plans dating back to the 1850s indicate minimal intervention management, and since 1925, Suserup Skov has been virtually unmanaged (Heilmann-Clausen et al. 2007). Despite an area of just 20 ha, around 800 species of fungi and lichens alone have been recorded in Suserup Skov, which highlights the remarkable biodiversity (Danish Mycological Society 2016).
However, the initiative should be considered only as a single step towards securing the forest biodiversity in Denmark. Based on scientific analyses, Petersen et al. (2016) estimated that a minimum of 75,000 ha of unmanaged broadleaved forest is needed for the long-term conservation of the majority of forest species in Denmark. It was stressed, however, that this would be an absolute minimum solution, which requires that the reserves are selected on the basis of the distribution of species; and in any case, a larger network will provide even better protection of the biodiversity. An important aspect of this is that only very little private forest is set aside in Denmark although it comprises about 75% of the Danish forest land. Thus, it is important that future initiatives specifically consider the need for forest reserves on private land.
Climate change regulation
The political decision to set aside more forests in Denmark was justified solely with regard to the conservation of biodiversity. However, the changed management of the forest will also to some extent affect the climate through changes in the uptake and emissions of CO2 and other greenhouse gasses (GHGs), and the storage of carbon. These changes will depend on the future management and the long-term biological development in the reserves, cf. the descriptions in the sections above.
Johannsen et al. (2020) has estimated the potential climate effects of the new forest reserves and these results are further discussed in Petersen et al. (2020). The overall conclusion was as follows: When the forests are left unmanaged, the carbon stock in the tree biomass will increase, which will contribute to climate change mitigation. The net uptake of CO2, and thus the increase of the carbon stock will slow down in the long term. A smaller contribution comes from changes in the forest soil by accumulation of carbon mainly in the long term and mainly through the rewetting of drained areas.
In more specific terms, it was estimated that the total carbon stock in the living and dead biomass in the future reserves would increase from initially 4460 kt CO2-eq (CO2-equivalents) to 4580–8450 kt in 2050 and 5290–9970 kt in 2100. At the same time, the accumulated emission of GHG’s from rewetted drained areas would be reduced to 176 kt CO2-eq from 331 kt in a business-as-usual scenario without rewetting (Johannsen et al. 2020). The carbon stock estimates were based on model calculations of the expected tree growth in scenarios with different assumption concerning (1) the maximum biomass levels in wooded areas to be reached after 200 years, and (2) the future management and ecosystem development determining mainly the openness of the forest. The highest carbon stock levels in the ranges given above were reached in scenarios with no logging even in the implementation phase, and maximum biomass levels in wooded areas similar to those found in the Danish long-term unmanaged forest like Suserup Skov, described above (Nord-Larsen et al. 2019). The lowest levels came out in scenarios with clearing of one third of the presently wooded areas and maximum biomass levels similar to those recorded in the currently oldest stands within the new designations. These are lower than in the long-term unmanaged stands. The estimated GHG emissions in rewetted drained areas was based IPCC standard values.
Despite a substantial uncertainty, the estimates above suggests that the unmanaged forest will contribute to climate mitigation, mainly due to an increased carbon stock in the biomass. Thus, the biomass may eventually more than double in the new Danish reserves. While this is an important finding at the general level, the specific initiative in itself represents a small contribution due to the modest total area to be set aside. The estimated additional carbon sequestration (uptake and storage) corresponds to less than 0.25% the total Danish CO2 emission in the years 2017–2050 and less than 0,1% in the years 2050–2100.
Another aspect of establishing the forest reserves is that wood products are no longer harvested, which could otherwise contribute to climate mitigation by substitution of fossil fuels and GHG-intensive materials like concrete and steel. The long-term significance of this is uncertain, however, and depends strongly on the development in other sectors, but a further discussion of this highly debated aspect is beyond the scope of this case study.
The overall conclusion is that carbon sequestration associated with setting aside 13,800 ha of Danish state forests for biodiversity conservation will provide a minor but permanent side benefit in terms climate change mitigation.
Evaluation of evidence
The prioritization and designation process of state forest reserves is well documented, through technical reports published by the two largest universities in Denmark (Copenhagen and Aarhus) and the Ministry of Environment and Food. There is not yet any direct evidence or quantitative estimates on the future effects of the planned effort on the biodiversity. However, there is a strong indirect evidence that it will considerably improve the conservation of forest biodiversity in Denmark.
Only few measurements of the carbon dynamics or the GHG balance of Danish forests are available. Furthermore, it is hard to reliably predict the future geophysical and biological development in the new forest reserves after logging is discontinued. However, the prediction of a positive climate mitigation effect due to carbon sequestration in the biomass and a minor positive contribution from the soil must be regarded as very certain, while the magnitude of these contributions are uncertain.
A particular issue worth mentioning here, is the general lack of scientific evidence from temperate broadleaved old growth forest, which is partly due to the diminutive distribution of such forests today, even at the European and global scales. In Denmark, this issue is reflected in fact that the small forest Suserup Skov serves a benchmark in almost any assessment or discussion of any aspect of leaving forest unmanaged for conservation. Examples of this are given above. Descriptions of structures and processes of this remarkable site has been compiled in Hahn & Emborg (2007), while other studies include e.g. Hannon et al. (2000), Heilmann-Clausen & Christensen (2004), Ódor et al. (2006), Ghalandarayeshi et al. (2017) and Nord-Larsen et al. (2019).
Synergy
The planned establishment of new forests reserves in Denmark will contribute significantly to biodiversity conservation at the national level. Thus, the initiative will more than double the forest area set aside for nature conservation in Denmark. In these areas more natural forest ecosystems will be restored and provide habitats for threatened species, in accordance with international agreements.
The initiative will also contribute to climate change mitigation through carbon sequestration (uptake and storage of CO2) mainly in the tree biomass, because the areas in general will no longer be logged. Although the purpose of the initiative is biodiversity conservation, this is an important finding in relation to the Paris climate agreement, which aims at carbon neutrality in all sectors.
Rewetting of drained areas is an important component in setting aside the state forest in Denmark. This is of particular significance to the biodiversity. Not only does it benefit species that are directly associated with wetlands, it also increases the spatial heterogeneity and the temporal dynamics of the forest in general. At same time, rewetting of drained soils will generally reduce the net emission of GHG’s in terms of CO2-equivalents. In the long term, these forest soils will often become net carbon sinks due to the accumulation of peat and humus.
6.8. Development of biodiversity and carbon storage in ancient boreal forests, Sweden
Introduction
This case study focuses on the terrestrial biodiversity and ecology on the islands in an archipelago in two adjacent lakes in the northern boreal forest zone of Sweden. Studies on these islands provide unique insights in the functioning of natural boreal forest ecosystems and the intimate coupling between biodiversity and carbon dynamics. The islands are forested and largely unaffected by humans, but subject to large scale disturbance from natural fires. Because the time since last fire ranges from less than 100 years to several thousands, the islands represent a unique system for studying the long-term development of a natural boreal forest ecosystem.
Through the last 25 years, these islands have been subject to extensive scientific studies addressing many different aspects of their functional ecology and biodiversity. Numerous studies have been published, many of which presents the most remarkable findings and hypotheses in high impact scientific journals including Science and Nature.
In addition to the findings on the islands, we look into some related research in managed forests in Northern and Central Sweden. Along with the Island studies, this research expands our understanding of the dynamics of biodiversity and carbon cycling in natural boreal ecosystems, and it highlights some possible implications for forest management.
Case
The islands are located within two adjacent lakes – Lake Hornavan and Lake Uddjaure – in the Northern Sweden (65°55’- 66°09’N; 17°43’- 17°55’E) (Fig. 6.8.1). The landscape is formed on morainic deposits created by the retreat of the ice sheets 9000 years ago (Wardle et al. 1997). Generally, the islands and islets are small, but vary in size. Most islands surveyed, ranges between 0.02 ha and 15 ha (corresponding to diameters of 16–400 m for circular islands).

Fig. 6.8.1 Lake Hornavan and Lake Uddjaure in Northern Sweden. The islands studied are generally too small to be shown on the map. (Map to the right is from Lantmäteriet).
The islands are forested and largely unaffected by human activities. Despite of this, many characteristics of the forest varies substantially across the islands, and it has turned out that the main driver of this variation is wildfire caused by lightning strike. As the islands were all formed at the same time just after the last glaciation, wild fire frequency is the only major external factor that differ among the islands. Large islands burn more often than smaller ones simply because they have a larger area to be intercepted by lightning. This means that some of the largest islands have burned in the past 100 years while some of the smallest have not burned for 5000 years (Clemmensen et al. 2013). Thus, there is a significant relationship between island size and the ecosystem developmental stage or “forest age”. Together, the islands form a post-fire chronosequence, which offer unique opportunities for studying the long-term development of undisturbed natural boreal forest. A chronosequence is a set of study sites that represent different ages of e.g. a forest ecosystem. The analysis of a chronosequence can be regarded as replacements, when true time series analyses of the same sites are not possible.
The most conspicuous consequence of the above pattern is that the plant species composition changes along the island gradient. Scots pine (Pinus sylvestris) and Bilberry (Vaccinium myrtillus) dominate on the large, more recently burned islands, Birch (Betula pubescens) and Lingonberry (Vaccinium vitis-idaea) dominate on mid-sized islands, and Norway spruce (Picea abies) and Crowberry (Empetrum hermaphroditum) dominate on small islands with the longest time since fire (Jonsson 2016). Examples of islands in the lakes are shown on Fig. 6.8.2.
The size-time-vegetation relationship was also found to be a major factor related to, or determining, several other ecosystem-level properties of the islands, including diversity and composition of fungi and animal communities, standing biomass, plant litter decomposition, nitrogen mineralization, carbon partitioning and turnover, humus accumulation, and plant nitrogen acquisition (Wardle et al. 1997, Wardle et al. 2012, Clemmensen et al. 2015, Jonsson et al. 2016).
Many of the studies referred to in this case study focus on comparative analyses of 30 selected islands divided into three groups: 10 “large” islands (>1.0 ha; mean time since last major fire 585 years), 10 “medium” islands (0.1 to 1.0 ha; mean time since fire, 2180 years), and 10 “small” islands (<0.1 ha; mean time since fire 3250 years). Across all the 30 islands, time since last fire ranged from 60 years to 5350 years (Wardle et al. 2012).
Land tenure and protection status
Most of the islands in Lake Uddjaure and southern Lake Hornavan are state owned, and accordingly, they are owned and managed by the state enterprise Sveaskog. Sveaskog owns 14% of forest land in Sweden and is Sweden's largest forest owner. Forestry with focus on wood production is the main business, but Sveaskog also develops the forest as a venue for nature-based experiences and works with nature conservation. The islands, however are not subject to any formal nature protection.
Generally, most of the Nordic boreal forest is privately owned but a substantial part is state owned and managed by state enterprises or agencies.
Current land use, human activities and stakeholders
Lake Hornavan and Lake Uddjaure are located in Northern Sweden in a region with a very low population density. The islands are uninhabited and largely unaffected by human activities. No active forest management or logging take place on the islands and the historical exploitation of wood is assumed to be negligible. The Islands are used only sporadically for outdoor recreation activities like camping and canoeing.
As owner of most of the islands, the state company Sveaskog is an important stakeholder, in addition to Arjeplog municipality and the local communities. However, the unique natural history of the islands also represents values of great importance to the wider society even at an international scale. During recent decades, scientific researchers of Swedish universities in particular have been among the most important stakeholders. At the general level, most Nordic boreal forest is managed as intensive plantation forestry aimed at wood production. Only very little primary old growth forest is left.

Fig. 6.8.2. Examples of the Islands studied in Lake Hornavan and Lake Uddjaure. Large island (top), medium sized island (middle) and small island (bottom) cf. the study design described in the text. Photos courtesy of Karina E. Clemmensen.
Climate change regulation
One of the most striking observations, is that total ecosystem carbon storage increases with time since fire across the chronosequence (Fig. 6.8.3). This shows that natural old growth forests may act as carbon sinks, taking up CO2 from the atmosphere potentially through several thousand years. The average total ecosystem carbon stock increased from about 11 kg per m2 at the 10 large islands (youngest forest) to 29 kg per m2 at the 10 small islands (oldest forest). However, this increase is only due to the belowground carbon stock, which went from 6.4 to 27 kg per m2 or from 60% to 95% of the total carbon pool. At the same time the above ground biomass carbon dropped from 45 to only 17 kg per m2 (Wardle et al. 2012). It should be noted that even at 10 the largest islands, the average time since last fire was 585 years, which is a very long time seen from a management perspective.
The above development of the carbon pools reflect that the ecosystem builds up and development occurs; but in the long-term absence of catastrophic disturbance, a biological decline phase eventually follows (Wardle et al. 2004). During this decline phase, or “retrogression”, plant production, biomass, nutrient availability and soil carbon turnover decrease. Analyses of five additional chronosequences found similar patterns of decline in forested ecosystems spanning the tropical, temperate, and boreal zones. Thus, it may be a general pattern that the maximal biomass phase reached during forest succession cannot be maintained in the long-term absence of major disturbance (Wardle et al. 2004).

Fig. 6.8.3. Carbon storage on islands in different size classes: L= large, M = medium and S = small. For size and mean time since last fire see text. Within each panel, bars topped by the same letter are not significantly different according to Tukey’s test at P = 0.05. (From Wardle et al. 2012)
The ecological role of fungi for ecosystem development
Additional studies of the islands have shown that fungi play a major role for the soil carbon dynamics and for the overall ecosystem development through time. First of all, it is estimated that 50–70% of the belowground carbon stored on the islands derives from roots and root-associated fungi (mycorrhizal fungi) (Clemmensen et al. 2013). This challenges the previous dogma that soil organic matter mainly arise from decomposition of aboveground plant litter.
Mycorrhiza refers to a symbiotic association between plants and soil fungi, which play important roles in the nutrition of the vast majority of plants including most boreal forest trees. In short, the plants supply sugars from the photosynthesis to the fungi, and the fungi supplies water and mineral nutrients from the soil to their host. This is rendered possible by the close physical contact between the fungal mycelium and the plant fine roots in the specialized structures called “mycorrhiza” (“fungus-root”, translated directly). Mycorrhiza is divided into two main forms; ectomycorrhiza, where the fungal mycelium forms a mantle around the root tips and only grows between the epidermal cells, and endomycorrhiza where the fungus penetrates the root cell walls and forms hyphal structures inside the root cells.
Mycorrhizal fungal communities are highly species-rich with several hundreds of species typically found in a single forest stand, including many commonly known mushrooms. Successional changes in the mycorrhizal fungal communities (i.e. changes in the species composition and dominance pattern over time) seem to drive the long-term carbon sequestration and the above and below ground carbon partitioning found with different forest developmental stages on the islands. The development can be summarized as follows (see Clemmensen et al. 2015): In the decades following a major fire, the primary production and tree growth is high. The trees invest much carbon (sugar) in their ectomycorrhizal symbionts, which also grow fast and acts as decomposers of soil organic matter. This process mobilizes organic nitrogen for the trees, and the soil organic carbon content decreases rather quickly. During later successional stages, the soil is gradually depleted of easily accessible nutrients; trees grow slower and the total allocation of carbon to mycorrhiza decreases; and ectomycorrhizal fungi decline, while more stress tolerant endomyccorrhizal ericoid fungi dominate. During this phase, impaired degradation and increased preservation of fungal residues explain the observed large buildup of soil carbon. This successional pattern is believed to be repeated after each major fire (Clemmensen et al. 2015).
Biodiversity
The long-term development of the boreal old growth forests on the islands in Lake Hornavan and Lake Uddjaure has direct implications for the biodiversity. First of all, it involves characteristic shifts of the vegetation over time. In the years after a major fire, vegetation is dominated by species profiting from the large and easy accessible nutrient pools in the soil. During the later stages, tree growth slow down, the above ground biomass fall, and more stress tolerant tree species and shrubs take over. (For dominating plant species see above). The ecosystem changes are also associated with substantial shifts in the communities of mycorrhizal fungi. This is of great importance in relation to biodiversity conservation as these fungi represent a large share of the biodiversity in boreal forests, and is crucial to ecosystem functioning. Finally, the shifts in vegetation is most likely a major factor determining the changes in diversity and composition of the animal communities (Wardle et al. 2012, Jonsson et al. 2016).
Further studies on the islands reveal that the time since last fire (ecosystem age) is of great importance for the diversity of several different species groups, and that different taxonomic and functional groups respond differently over time. Thus, the variation among islands (β-diversity) show almost any pattern such as increasing (litter fungi and nematodes), decreasing (beetles and vascular plants), hump-shaped (epiphytic lichens and root fungi) and U-shaped (spiders). The local species diversity at each island (α-diversity), also changes through time, but for some groups the pattern deviates substantially from the diversity among the islands (Jonsson et al. 2016).
These observations demonstrate that effective biodiversity conservation approaches should consider the natural variation at landscape level. The effort should focus on preserving not only the main ecosystem types, but also the full array of ecological development phases and changing biological communities that is found in the virgin boreal forest (Jonsson et al. 2016). Thus, the maximum effect on biodiversity is achieved by conserving large areas allowing natural disturbance and dynamics to occur.
Implications for managed forests
A characteristic succession of ectomycorrhizal fungi also takes place in managed boreal forests. This has been shown in a chronosequence of managed forest close to Uppsala in central Sweden. The study included Scots pine (Pinus sylvestris) stands ranging in age from 1 to 158 years. As in the natural ecosystems this successional pattern involves changes in soil and nutrient turnover and most likely also tree growth. In young stands, trees rely mainly on easily accessible nutrients released during decomposition and mineralization induced by the logging disturbance. However, in older stands, the ectomycorrhizal fungal communities mobilize nutrients through the decomposition of organic matter deeper in the soil (Kyachenko et al. 2017a, 2017b, Hagenbo et al. 2017). Thus, the role of root associated fungi as decomposers, explains their importance for tree growth, after the early and easily accessible nutrient pools are depleted.
Clear cutting of entire stands is still today the most common practice in boreal coniferous forestry, although it entails major impacts on the biodiversity. As ectomycorrhizal fungi are associated with the roots of living trees it is certainly a group of organisms adversely affected. Experimental clear cutting in an old unmanaged Scots pine forest in the northern Sweden showed that only the most common species survived locally (Sterkenburg et al. 2019). Other studies show that the recovery is slow. An analysis based on several different studies reports that species richness of ectomyccorhizal fungi takes, on average, 90 years to recover to old-growth forest levels after clearcutting (Spake et al. 2015).
In continuation of the above issue, attention has been drawn to the risk that repeated clearcuttings will affect forestry in the long term, in terms of reduced tree growth and wood production or increased need for fertilization. Considering the importance of mycorrhiza for the nutrient uptake and the carbon allocaton in the soil, also in managed forests, this could be the consequence if the abundance and diversity of ectomyccorhizal fungi are permanently reduced. Here it can be added that the ectomycorrhizal fungi that decompose and mobilize nitrogen in old forests are probably also sensitive to fertilization (Sterkenburg et al. 2015, Kyachenko et al. 2017b).
Retention forestry is a way of reducing logging impacts and enhancing biodiversity conservation. The approach is to retain structural features of old forests such as live and dead trees of varying sizes on logged areas. Such structures are important because they emulate “biological legacies” that are generally found in stands following natural disturbances (Franklin et al. 1997). In relation to ectomyccorrhizal fungi, the function of tree retention would be to “life-boat” the species through the logging and regeneration phase. A review of more than 200 European and North American studies showed that birds and ectomycorrhizal fungi benefited most from tree retention (Rosenvald & Lohmus 2008). However, the experimental clearcutting mentioned above shows that retention of at least 30–60% of the trees is needed to preserve most of the ectomycorrhizal diversity (Sterkenburg et al. 2019). This is much more than what is required by the most common sustainable forest management certification schemes today.
Evaluation of evidence
The ecosystems and the issues of this case study are generally well described. Most information is based on peer reviewed papers, several of which are published in leading scientific journals.
On the other hand, the pioneering nature of the discoveries means that the possible general significance of some of the observations and derived hypotheses are still uncertain. Even though the remarkable long term carbon sequestration on the islands is certainly relevant in the context of climate change mitigation, care should be taken to generalize from these observations. Thus, the properties of the forest ecosystem on the largest and most recently burnt islands are probably those most similar to the mainland forest. Zacrisson (1977) has estimated the natural frequency of fires in Scandinavian boreal fores forest to be 80 years, which is far less than the average time since last fire even on the “large” islands, with the youngest ecosystems, of the island studies.
Synergy
- The finding that even very old undisturbed boreal forest may act as a carbon sink does in it-self represent an important synergy between biodiversity conservation and climate change mitigation. The importance of old growth forest for biodiversity conservation is well known and well-documented. When it comes to the possible synergies with climate change mitigation, it has been vigorously debated for more than a decade, to what extent old growth forests acts as carbon sinks. The islands of this case study represent one of the most remarkable examples of this being the case.
- The observed biodiversity patterns at the islands support this possible synergy. Thus, Wardle et al. (2012) state that “We show that taxonomic richness of plants and above-ground consumers are positively correlated with total ecosystem C storage, suggesting that conserving old-growth forests simultaneously maximizes biodiversity and C sequestration”. There is little evidence, though, that biodiversity in it-self increase the carbon sequestration on the islands. The patterns of both parameters should rather be seen as consequences of the underlying changes of the ecosystem through time, including shifts in vegetation and carbon and nutrient turnover and partitioning between ecosystem compartments.
In managed boreal forests, focus on the preservation of ectomycorrhizal fungi may also represent a synergy with climate change mitigation in the long term due to their importance for tree growth. Thus, if loss of fungal diversity actually reduces tree growth, it may in turn limit the contribution of harvested wood products to climate mitigation through carbon storage and substitution of fossil fuels and energy intensive materials like concrete and steel.
Acknowledgement
We thank Karina Engelbrecht Clemmensen at the Swedish University of Agricultural Sciences for providing important knowledge and literature, for valuable comments on the manuscript and for photos of the Islands.
7. Acknowledge|ment
We are grateful to Sigga Jacobsen, Lajla Tunaal White, Eva Juul Jensen and Agnes Brá Birgisdóttir, Nordic Working Group on Biodiversity for input and advice to the project and to Hans Joosten, Griefswald University for generous support with information on peatland issues. We thank Louise Imer Nabe-Nielsen, Linn Naturraadgivning for going through the final text. Finally, we are grateful to all the people that kindly helped us with case studies by sharing their knowledge and relevant literature. Names are listed in the acknowledgment sections in each case study.
References
ACIA 2005. Arctic Climate Impact Assessment. Cambridge University Press, 1042 pp.
Allen K. A., Lehsten V., Hale K., Bradshaw R. H. W. 2016. Past and future drivers of an unmanaged carbon sink in European temperate forest. Ecosystems. 19:545–554.
An, S., Zong, X. 2016. The 10th INTECOL International Wetlkands Conference. Hotspots of Biodiversity and Ecosystem Services under Global Changes. Changshu, China.
Anonymous 2001. Biological Diversity in Iceland. National Report to the Convention on Biological Diversity. Ministry for the Environment and the Icelandic Institute of Natural History. Reykjavík. 56 pp.
Anonymous 2014. Iceland. The fourth national report to the Convention of Biological Diversity. Ministry for the Environment and National Resources. Reykjavík. 39 pp.
Anonymous, 2015. Rapport över uppföljningen inom Life to ad(d)mire G-län. Kronobergs Län.
Aradóttir, A. L. 2007. Restoration of birch and willow woodland on eroded areas. In: Effects of afforestation on ecosystems, landscape and rural development. Tema Nord 2007:508 Nordic Council of Ministers, Copenhagen.
Aradóttir, A. L and Eysteinsson, T. 2005. Restoration of birch woodlands in Iceland. In: Stanturf, J. A. and Madsen, P. (eds.) 2005. Restoration of boreal and temperate forests. CRC Press.
Aradottir, A. L., Halldorsson, G. 2018. Colonization of woodland species during restoration: seed or safe site limitation? Restoration Ecology 26: 73-83.
Aradóttir, A. L., Petursdottir, T., Halldorsson, G., Svavarsdottir, K., Arnalds, O. 2013. Drivers of Ecological Resto-ration: Lessons from a Century of Restoration in Iceland. Ecology and Society 18.
Arnalds, O. 2015. The Soils of Iceland. World Soils Book Series, Springer, Dordrecht. 183 pp.
Bachmore, D. 2018. Ecosystem Services at Lille Vildmose - Quantification of carbon sequestration, biodiversity and tourism following restoration measures. Master Thesis project, University of Copenhagen.
Bader, P. 2019. Uppföljning av Stensjöflon naturreservat - inventering av myrfåglar 2017 Länsstyrelsen Västernorrland.
Bárcena, T. G., Kiær, L. P., Vesterdal, L., Stefánsdóttir, H. M., Gundersen, P., Sigurdsson, B. D. 2014. Soil carbon stock change following afforestation in Northern Europe: a meta-analysis. Global Change Biology 20: 2393–2405.
Barfod, A. S., Bruun, H. H., Clausen, P., Dinesen, L., Egemose, S. et al. 2020. Genopretning af Biodiversitet og Økosystemer. – Det danske IPBES-kontor.
Barthelmes, A., Couwenberg, J., Risager, M., Tegetmeyer, C. and Joosten, H. 2015. Peatlands and Climate in a Ramsar context: A Nordic-Baltic Perspective. Nordic Council of Ministers and Ramsar NorBalWet, Denmark. 244 pp.
Bartlett, J., Rusch, G. M., Kyrkjeeide, M. O., Sandvik, H., Nordén, J. 2020. Carbon storage in Norwegian ecosystems. NINA Report 1774b. Norwegian Institute for Nature Research.
Behrend, A. B. 2019. Natural succession after heavy grazing pressure. A case study of the birch forests of Þórsmörk and Goðaland, southern Iceland. M.Sc. thesis. Department of Geosciences and Natural Resource Management, University of Copenhagen. 55 p.
Berg, Å., Ehnström, B., Gustafsson, L., Hallingbäck, T., Jonsell, M., Weslien, J. 1994. Threatened plant, animal, and fungus species in Swedish forests: distribution and habitat associations. Conserv. Biol. 8, 718–731
Berglund, B.E. (ed.) 1991. The cultural landscape during 6000 years in southern Sweden – the Ystad project. Ecological Bulletins 41: 1–495.
Bonn, A., Allott, T., Evans, M., Joosten, H., & Stoneman, R. (Eds.). 2016. Peatland Restoration and Ecosystem Services: Science, Policy and Practice (Ecological Reviews). Cambridge: Cambridge University Press.
Borge, A. F., Westermann, S., Solheim, I., Etzelmüller, B. 2017. Strong degradation of palsas and peat plateaus in northern Norway during the last 60 years. The Cryosphere, 11: 1–16.
Braun, M., Fritz, D., Braschel, N., Büchsenmeister, R., Freudenschuss, A., Gschwantner, T., Jandl, R., Ledermann, T., Neumann, M., Pölz, W., Schadauer, K., Schmid, C., Schwarzbauer, P., Stern, T., Weiss, P. 2016. A holistic assessment of green house gas dynamics from forests to the effects of wood products use in Austria. Carbon Management 7, 271–283.
Bryn, A., Dourojeanni, P., Hemsing, L. Ø., O'Donnell, S. 2013. A high-resolution GIS null model of potential forest expansion following land use changes in Norway, Scandinavian Journal of Forest Research, 28:1, 81-98.
Camill, P., Jason, A., Lynch, J. S., Clark, J., Adams, B. 2014. Changes in Biomass, Aboveground Net Primary Production, and Peat Accumulation following Permafrost Thaw in the Boreal Peatlands of Manitoba, Canada. Ecosystems 4, 461–478.
CBD 2020. Preparations for the Post-2020 Biodiversity Framework. Convention on Biological Diversity. https://www.cbd.int/conferences/post2020
Christensen, M. & Emborg, J. 1996. Biodiversity in natural versus managed forest in Denmark. Forest Ecology and Management, 85, 47-51.
Ciais P., Schelhaas M.J., Zaehle S., Piao S. L., Cescatti A., Liski J., Le-Marie, G., Schulze, E.D., Bouriaud, O., Freibauer, A., Valantini, R., Nabuurs, G.J. 2008. Carbon accumulation in European forests. Nature Geoscience 1, 425-429.
Clemmensen K. E., Bahr A., Ovaskainen O., Dahlberg A., Ekblad A., Wallander H., Stenlid J., Finlay R. D., Wardle D. A., Lindahl B. D. 2013. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339: 1615–1618.
Clemmensen, K. E., Finlay, R. D., Dahlberg, A., Stenlind, J., Wardle, D. A., Lindahl, B. D. 2015. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytologist 205: 1525–1536.
Council of Europe 2015. Interpretation manual of the habitats listed in Resolution No. 4 (1996) listing endangered natural habitats requiring specific conservation measures. Convention on the conservation of European wildlife and natural habitats. Group of Experts on Protected Areas and Ecological Networks.
Crouzeilles, R., Curran, M., Ferreira, M. S., Lindenmayer, D. B., Grelle, C.E., & Rey Benayas, J. M. 2016. A global meta-analysis on the ecological drivers of forest restoration success. Nature Communications, 7, 11666.
Danish Mycological Society, 2016. Danish fungal records database, contributed, maintained and validated by Frøslev, T., Heilmann-Clausen, J., Lange, C., Læssøe, T., Petersen, J.H., Søchting, U., Jeppesen, T.S., Vesterholt, J†. Online: www.svampeatlas.dk.
Davidson, N. C., Dinesen, L., Fennessy, S., Finlayson, C. M., Grillas, P., Grobicki, A., McInnes, R. J., Stroud, D. A. 2019. A review of the adequacy of reporting to the Ramsar Convention on change in the ecological character of wetlands. Marine and Freshwater Research 70:11.
Dinesen, L., Hahn, P. 2019. Draft Ramsar Technical Report on peatland restoration and rewetting methodologies in Northern bogs. Ramsar Convention. Scientific and Technical Review Panel.
Eggermont, H., Balian, E., Azevedo, J. M. N., Beumer, V., Brodin, T., Claudet, J., Fady, B., Grube, M., Keune, K., Lamarque, P., Reuter, K., Smith, M., van Ham, C., Weisser, W., Le Roux, X. 2015. Nature-based Solutions: New Influence for Environmental Management and Research in Europe. GAIA 24:243-48.
Elmarsdottir A. and Magnusson, B. 2007. ICEWOODS: Changes in ground vegetation following afforestation. In: Halldorsson, G., Oddsdottir, E.S. & Eggertsson, O. (eds.) 2007. Effects of afforestation on ecosystems, landscape and rural development. TemaNord 2007:508. Nordic Council of Ministers, Copenhagen.
Elmarsdottir, A., Sigurdsson, B. D., Magnusson, B., Gudleifsson, B. E., Oddsdottir, E. S., Olafsson, E., Halldorsson, G., Eyjolfsdottir, G. G., Skarphedinsson, K. H., Ingimarsdottir, M., Nielsen, O. K. 2007. ICEWOODS: Age–related dynamics in biodiversity and carbon cycling of Icelandic woodlands. Experimental design and site descriptions. In: Halldorsson, G., Oddsdottir, E. S. and Eggertsson, O. (eds.) 2007. Effects of afforestation on ecosystems, landscape and rural development. TemaNord 2007:508 Nordic Council of Ministers, Copenhagen.
Elmarsdottir, A., Sigurdsson, B. D., Oddsdottir, E. S., Fjellberg, A., Gudleifsson, B. E., Magnusson, B., Olafsson, E., Halldorsson, Guðmundsson, G. A., Eyjolfsdottir, G. G., Skarphedinsson, K.H., Ingimarsdottir, M., Nielsen, O. K. 2011. Áhrif skógræktar á tegundaauðgi [Effects of afforestation on species richness] Náttúrufræðingurinn 81: 69–81.
Elsgaard, L. 2018. Carbon sequestration in bogs and the importance of peatlands in a climate perspective. LIFE+ Lille Vildmose final conference. Comwell Rebild Bakker.
EU Commission 2020. EU Biodiversity Strategy for 2030. https://ec.europa.eu/environment/nature/biodiversity/strategy/index_en.htm
Finnish Forest Research Institute 2014. http://www.metla.fi/index-en.html
Flugsrud, K., Økstad, E., Kvissel, O.-K., Backer, E. B., Søgaard, G., Granhus, A., Terum, T., Bøe, L. V. 2016. Vern eller bruk av skog som klimatiltak. Rapport M519/2016, Norwegian Environment Agency, Norwegian Agriculture Agency, Norwegian Institute of Bioeconomy Research.
Framstad, E., Stokland, J. N., Hylen, G. 2011. Skogvern som klimatiltak. Verdifulle skogtyper for biologisk mangfold og karbonlag-ring – NINA Rapport 752. 38 s.
Framstad, E., Wit, H., Mäkipää, R., Larjavaara, M., Vesterdal, L., Karltun, E. 2013. Biodiversity, carbon storage and dynamics of old northern forests. Nordic Council of Ministers. TemaNord 2013:507. 130 pp.
Franklin, J. F., Berg, D. R., Thornburgh, D. A., Tappeiner, J. C. 1997. Alternative silvicultural approaches to timber harvesting: variable retention harvest systems. In: Kohm, K.A. and Franklin, J.F. (Eds.): Creating a Forestry for the 21st Century: The Science of Ecosystem Management. Island Press, Washington, D.C., pp. 111–139.
Fritzbøger, B. & Odgaard, B. 2017. Skovenes historie [The history of the forest] pp. 55-88 in Møller, P. F. (red.). Naturen i Danmark: Skovene [The Nature in Denmark]. 2nd edition. Gyldendal.
Frolking, S., Roulet, N. T. 2007. Holocene radiative forcing impact of northern peatland carbon accumulation and methane emissions. Global Change Biology 13: 1079–1088.
Ghalandarayeshi S., Nord-Larsen T., Johannsen V. K., Larsen J.B. 2017. Spatial patterns of tree species in Suserup Skov - a semi-natural forest in Denmark. Forest Ecology and Management 406: 391-401.
Griscom, B. W., Adams, J., Ellis, P. W., Houghton, R. A., Lomax, G., Miteva, D. A., Schlesinger, W. H., (...), Fargione, J. 2017. Natural climate solutions. Proceedings of the National Academy of Sciences 114: 11645-11650.
Gunnarsson, U. & Löfroth, M. 2014. The Swedish Wetland Survey – compiled excerpts from the national final report. Naturvårdsverket Rapport 6618.Götbrink, E. 2015. Uppföljning av Taglamyren och Flymossen före och efter restaurering En undersökning inom ramen för Life to ad(d)mire, Kronobergs Län. Kråkfot Natur AB.
Günther, A., Barthelmes, A., Huth, Y., Joosten, H., Jurasinski, G., Koebsch, F., Couwenberg, J. 2019. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nature Communications 1-5.
Götbrink, E. 2015. Uppföljning av Taglamyren och Flymossen före och efter restaurering En undersökning inom ramen för Life to ad(d)mire, Kronobergs Län. Kråkfot Natur AB.
Götmark, F. 2013. Habitat management alternatives for conservation forests in the temperate zone: Review, synthesis, and implications. Forest Ecology and Management 306: 292–307.
Hagenbo, A., Clemmensen, K. E., Finlay, R. D., Kyaschenko, J., Lindahl, B. D., Fransson, P., Ekblad, A. 2017. Changes in turnover rather than production regulate biomass of ectomycorrhizal fungal mycelium across a Pinus sylvestris chronosequence. New Phytologist 214: 424–431.
Hahn, K., Emborg, J. (Eds.) 2007. Suserup Skov: Structures and Processes in a Temperate, Deciduous Forest Reserve. Ecological Bulletins 52. 200 pp.
Hahn, P. 2015. Peatlands Climate regulation and biodiversity. Naturstyrelsen. https://www.youtube.com/watch?v=ZcxZ9gvNfSU
Halldorsson, G., Oddsdottir E.S., Eggertsson, O. (eds.). 2007. Effects of afforestation on ecosystems, landscape and rural development. Proceedings of the AFFORNORD conference, Reykholt, Iceland, June 18–22, 2005. TemaNord 2007:508 Nordic Council of Ministers, Copenhagen.
Hallanaro, E., Pylvänäinen, M. 2001. Nature in Northern Europe – Biodiversity in a changing environment. Nordic Council of Ministers. Nord. Copenhagen 349 pp.
Hannon, G. E., Bradshaw, R., Emborg, J. 2000. 6000 years of forest dynamics in Suserup Skov, a seminatural Danish woodland. Global Ecology and Biogeography 9: 101-114.
Hansen, K. 2011. Folk og Fortællinger fra Det Tabte Land. Bind I. Jylland. Forlaget Bæredygtighed.
Harmon, M. E., Ferrell, W. K., Franklin, J.F. 1990. Effects on carbon storage of conversion of oldgrowth forests to young forests. Science 247:699–702.
Heilmann-Clausen, J., Christensen, M. 2004. Does size matter? On the importance of various dead wood fractions for fungal diversity in Danish beech forests. For. Ecol. Manag. 201: 105–117.
Heilmann-Clausen, J., Bradshaw, R. H. W., Emborg, J., Hannon, G. 2007. The history and present conditions of Suserup Skov - a nemoral, deciduous forest reserve in a cultural landscape. Ecological Bulletins 52: 7-17.
Hofgaard, A. 2003. Effects on climate change on the distribution and development of Palsa peatlands: Background and suggestions for a national monitoring project. Norwegian Institute Nature Research.
Hofgaard, A. 2019. Monitoring Palsa mires in Norway. In Proceedings and Abstracts International meeting on “Mires and Wetlands of the North Calotte”. Nordkalottrådet.
Hofgaard, A., Kyrkjeeide, M. O. & Myklebost, H. E. 2020. Overvåking av palsmyr. Tredje gjenanalyse i Ostojeaggi, Troms. Endringer fra 2004 til 2019. NINA Rapport 1920. Norsk institutt for naturforskning.
Hudson, L. N. et al. 2014. The PREDICTS database: a global database of how local terrestrial biodiversity responds to human impacts. Ecology & Evolution 4, 4701-4735.
Hudson L. N. et al. 2016. The database of the PREDICTS (Projecting Responses of Ecological Diversity In Changing Terrestrial Systems) Project. Ecology & Evolution.
Hugelius, G., Strauss, J., Zubrzycki, S., Harden, J.W., Schuur, E. A. G., Ping, C.-L., Schirrmeister, L., Grosse, G., Michaelson, G. J., Koven, C. D., O’Donnell, J. A., Elberling, B., Mishra, U., Camill, P., Yu, Z., Palmtag, J. & Kuhry, P. 2014. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences, 11, 6573–6593.
Humpenöder, F., Karstens, K., Lotze-Campen, H., Leifeld, J., Menichetti, L., Barthelmes, A., Popp, A. 2020. Peatland protection and restoration are key for climate change mitigation. Environmental Research Letters.
Häkkinen, M., Heikkinen, J. & Mäkipää, R. 201. Soil carbon stock increases in the organic layer of boreal middle-aged stands. Biogeoscience 8: 1279–1289.
IPBES 2018. Summary for policymakers of the assessment report on land degradation and restoration of the Intergovernmental Platform on Biodiversity and Ecosystem Services. IPBES Secretariat, Bonn, Germany. 44p.
IPBES 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Platform on Biodiversity and Ecosystem Services. IPBES Secretariat, Bonn, Germany. 56p.
IPBES 2020. Resources. Core Glossary: https://ipbes.net/glossary?combine=biodiversity
IPCC 2014. 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands. Hiraishi, T., Krug, T., Tanabe, K., Srivastava, N., Baasansuren, J., Fukuda, M. and Troxler, T.G. (eds.). IPCC, Switzerland.
IPCC 2018. Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty (ed. by V. Masson-Delmotte, P. Zhai, H. O. Pörtner, D. Roberts, J. Skea, P.R. Shukla, A. Pirani, W., Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. R. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, T. Waterfield). IPCC, Geneva.
IPCC 2019. Summary for Policymakers. In: Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems [P.R. Shukla, J. Skea, E. Calvo Buendia, V. Masson-Delmotte, H.- O. Pörtner, D. C. Roberts, P. Zhai, R. Slade, S. Connors, R. van Diemen, M. Ferrat, E. Haughey, S. Luz, S. Neogi, M. Pathak, J. Petzold, J. Portugal Pereira, P. Vyas, E. Huntley, K. Kissick, M. Belkacemi, J. Malley, (eds.)].
Ísleifsson, Ó. Eggertsson 2016. Náttúrulegt birki á Íslandi – ný úttekt á útbreiðslu þess og ástandi. [Natural birch wood-land in Iceland – a new assessment on distribution and state]. Náttúrufræðingurinn 86, 97-111.
IUCN 2019. Nature-based solutions in nationally determined contributions. Gland : IUCN ; Oxford : University of Oxford.
Jauhiainen, J., Alm, J., Bjarnadottir, B., Callesen, I. et al. 2019. Reviews and syntheses: Greenhouse gas exchange data from drained organic forest soils – a review of current approaches and recommendations for future research. Biogeosciences, 16, 4687–4703.
Johannsen, V. K., Nord-Larsen, T. 2020. Klimaeffekter af urørt skov og anden biodiversitetsskov 2020. [Climate effects of unmanaged forest and other biodiversity forest.] Sagsnotat. 44 pp. (Note in Danish).
Johnston, C.E. et al 2014. Effect of permafrost thaw on CO2 and CH4 exchange in a western Alaska peatland chronosequence. Environ. Res. Lett. 9 085004.
Jones, M. C., Harden, J., O’donnell, J., Manies, K., Jorgenson, T., Treat, C., Ewing, S. 2017. Rapid carbon loss and slow recovery following permafrost thaw in boreal peatlands. Global Change Biology 23: 1109–1127.
Jonsson, M., Snäll, T., Asplund, J., Clemmensen, K. E., Dahlberg, A., Kumordzi, B. B., Wardle, D. A. 2016. Divergent responses of b-diversity among organism groups to a strong environmental gradient. Ecosphere 7: 1–13.
Jónsson, T. H. 2001, Case study: Native Birch woods in Iceland. Box p 44 in: Anonymous 2001. Biological Diversity On Iceland. National Report to the Convention on Biological Diversity. Ministry for the Environment and The Icelandic institute of Natural History. Reykjavík 56 pp.
Jónsson, T. H. 2004. Stature of Sub-arctic Birch in Relation to Growth Rate, Lifespan and Tree Form. Annals of Botany 94: 753-762.
Joosten, H. 2009. The Global CO2 Picture. Peatland status and drainage associated emissions in all countries of the World. Wetlands International.
Joosten, H., Sirin, A., Couwenberg, J., Laine, J., Smith, P. 2018. The role of peatlands in climate regulation. In Peatland Restoration and Ecosystem Services: Science, Policy and Practice, eds. Bonn, A., Allott, T., Evans, M., Joosten, H., Stoneman, R. Cambridge University Press.
Joosten, H., Tanneberger, F., Moen, A. 2015. Mires and peatlands of Europe. Status, distribution and conservation. Schweizerbart Science Publishers. Stuttgart.
Joosten, H. in press. 2020. Ramsar Global Guidelines for Peatland Rewetting and Restoration. Ramsar Technical Report. Juutinen, A., Saarimaa, M., Ojanen, P., Sarkkola, S., Haara, A., Karhu, J., Nieminen, M., Minkkinen, K., Penttilä, T., Laatikainen, M. & Tolvanen, A. 2019. Trade‐Offs between Economic Returns, Biodiversity, and Ecosystem Services in the Selection of Energy Peat Production Sites. Ecosyst. Serv. 40: 101027.
Juutinen, A., Tolvanena, A., Saarimaa, M., Ojanen, P., Sarkkola, S., Ahtikoski, A., Haikarainen, S., Karhu, J., Haara, A., Nieminen, M., Penttilä, T., Nousiainen, H., Hotanen, J-H., Minkkinen, K., Kurttila, M., Heikkinen, K., Sallantaus, T., Aapala, K., Tuominen, K., 2020. Cost-effective land-use options of drained peatlands– integrated biophysical economic modeling approach. Ecological Economics 175. 106704. https://www.sciencedirect.com/journal/ecological-economics
Karki, S., Elsgaard, L., Kandel, T. P., Lærke, P. E. 2016. Carbon balance of rewetted and drained peat soils used for biomass production: A mesocosm study. GCB Bioenergy 8: 969–980.
Karlsdóttir, L. 2014. Hybridisation of Icelandic birch in the Holocene reflected in pollen. Ph.D. thesis. Faculty of Life and Environmental Sciences, School of Engineering and Natural Sciences, University of Iceland. Reykjavik. 148 pp.
Keller, N., Stefani, M., Einarsdóttir, S.R., Helgadóttir, Á.K., Guðmundsson, J., Snorrason, A., Þórsson, J., Tinganelli, L. 2020. National Inventory Report Emissions of greenhouse gases in Iceland from 1990 to 2018. The Environment Agency of Iceland. 312 pp.
Kettridge, N., Turetsky, M. R., Sherwood, J. H., Thompson, D. K., Miller, C. A., Benscoter, B. W., Flannigan, M. D., Wotton,. B. M., Waddington, J. M. 2015. Moderate drop in water table increases peatland vulnerability to post-fire regime shift. Scientific reports 5: 8063.
Klapstein, S. J., M. R. Turetsky, A. D. McGuire, J. W. Harden, C. I. Czimczik, X. Xu, J. P. Chanton, and J. M. Waddington 2014, Controls on methane released through ebullition in peatlands affected by permafrost degradation, J. Geophys. Res. Biogeosci., 119, 418–431.
Koven, C. D., Ringevala, B., Friedlingsteinc, P., Ciaisa, P., Cadulea, P., Khvorostyanovd, D., Krinnere, G., Tarnocaif, C. 2011. Permafrost carbon-climate feedbacks accelerate global warming. PNAS 108 no. 36: 14769–14774.
Kurttila, M., Haara, A., Juutinen, A., Karhu, J., Ojanen, P., Pykäläinen, J., Saarimaa, M., Tarvainen, O., Sarkkola, S. & Tolvanen, A., 2020. Applying a Multi‐Criteria Project Portfolio Tool in Selecting Energy Peat Production Areas. Sustainability 12: 2-16.
Kyaschenko, J., Clemmensen, K. E., Hagenbo, A., Karltun, E., & Lindahl, B. D. 2017a. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME Journal 11: 863-874.
Kyaschenko J, Clemmensen K. E., Karltun E., Lindahl B. D. 2017b. Below-ground organic matter accumulation along a boreal forest fertility gradient relates to guild interaction within fungal communities. Ecology Letters 20: 1546–1555.
Lawrence, D. M., Slater, A. G. 2005. A projection of severe near-surface permafrost degradation during the 21st century. Geophysical Research Letters 32.
Leifeld, J., Alewell, C., Bader, C., Krüger, J. P., Mueller, C. W., Sommer, M., Steffens, M., Szidat, S. 2018. Pyrogenic carbon contributes substantially to carbon storage in intact and degraded northern peatlands. Land Degradation and Development 29: 2082–2091.
Leifeld, J., Wüst-Galley, C. & Page, S. 2019. Intact and managed peatland soils as a source and sink of GHGs from 1850 to 2100. Nature Climate Change 9: 945–947.
Lelli, C., Bruun, H. H., Chiarucci, A., Donati, D., Frascaroli, F., Fritz, Ö., Goldberg, I., Nascimbene, J., Tøttrup, A., Rahbek, C., Heilmann-Clausen, J. 2018. Biodiversity response to forest structure and management: Comparing species richness, conservation relevant species and functional diversity as metrics in forest conservation. For. Ecol. Manage. 432, 707–717.
Leskinen, P., G. Cardellini, S. González-García, E. Hurmekoski, R. Sathre, J. Seppälä, C.Smyth, T. Stern and P.J. Verkerk 2018. Substitution effects of wood-based products in climate change mitigation. From Science to Policy 7. European Forest Institute.
LIFE 2016. FINAL Report LIFE to ad(d)mire. Covering the project activities from 01/01/2010-31/12/2015. LIFE08/NAT/S/000268.
LIFE 2018. Final Report (shortened version) of the EU LifePeatlandUse project. Covering the project activities from 01.07.2013 to 30.6.2018. Natural Resources Institute Finland (Luke).
Lindenmayer, D. B., Franklin, J. F., Fischer, J. 2006. General management principles and a checklist of strategies to guide forest biodiversity conservation. Biological Conservation, 131, 433–445.
Lindholm, T., Heikkia, R. 2017. Finland. In Joosten, H., Tanneberger, F. & Moen, A. (Eds.) 2017. Mires and peatlands of Europe – status, distribution and conservation. Schweizerbart Science Publishers, Stuttgart.
Liski, J., Ilvesniemi, H., Mäkelä, A., Starr, M. 1998. Model analysis of the effects of soil age, fires and harvesting on the carbon storage of boreal forest soils. European Journal of Soil Science 49: 407–416.
Löfroth, M. 2001. Våtmarkernas situation och långsiktiga förändringar. In: Agerlind, G. (ed.), Landskapet: restprodukt eller medvetet skapat? Kungl. skogs och lantbruksakademins tidsskrift 140: 47–60.
Löfroth, M. 2015. Sweden. In: Joosten H., Tanneberger F., Moen, A. (eds.), Mires and peatlands of Europe – Status, distribution, and nature conservation, Schweizerbart Science Publishers, Stuttgart, Germany.
Markkula, I., Turunen, M., Rasmus, S. 2019. A review of climate change impacts on the ecosystem services in the Saami Homeland in Finland. Science of the Total Environment 692: 1070–1085.
Marteinsdottir, B., Svavarsdottir, K., and Þórhallsdóttir, Þ. E. 2007. Landnám birkis á Skeiðarársandi [Birch colonisation on Skeiðarársandur, SE-Iceland]. Náttúrufræðingurinn 75: 123-129.
Martin, L. C. P., Nitzbon, J., Aas, K. S., Etzelmüller, B., Kristiansen, H., Westermann, S. 2019. Stability conditions of peat plateaus and palsas in northern Norway. Journal of Geophysical Research: Earth Surface: 124 705–719.
Marushchak, M. E., Pitkämäki, A., Koponen, H., Biasi, C., Seppälä & Martikainen, P.J. 2011. Hot spots for nitrous oxide emissions found in differentes of permafrost peatlands. Global Change Biology 17: 2601–2614.
Ministry for the Environment. 2007. Iceland’s Climate Change Strategy. Ministry for the Environment. Reykjavík. 37 pp.
Ministry of Agriculture and Forestry, 2012. Government decision on the sustainable use and protection of mires and peatlands (in Finnish).
Ministry of Economic Affairs and Employment of Finland, 2013. National Energy and Climate strategy.
Ministry of Economic Affairs and Employment of Finland, 2014. Energy and climate roadmap 2050. MEE Publications 31/2014 (71 pp).
Moen, A. 1998. Vegetasjonsatlas for Norge. Hønefoss: Statens kartverk.
Myhre, B., Øye, I. 2002. Norges landbrukshistorie 1 [The agricultural history of Norway 1]. Det norske samlaget. 495 pp.
Müller, J. & Bütler, R. 2010. A review of habitat thresholds for dead wood: a baseline for management recommendations in European forests. European Journal of Forest Research 129: 981–992.
Mäkipää, R., Linkosalo, T., Niinimäki, S., Komarov, A., Bykhovets, S., Tahvonen, O. & Mäkelä, A. 2011. How forest management and climate change affect the carbon sequestration of a Norway spruce stand. Journal of Forest Planning 16, 107–120.
Nabuurs, G.-J., P. Delacote, D. Ellison, M. Hanewinkel, L.Hetem€aki, and M. Lindner. 2017. By 2050 the mitigationeffects of EU forests could nearly double through climatesmart forestry. Forests 8:484.
National Forestry Accounting Plan for Finland 2018. Ministry of Agriculture and Forestry, Finland.
Naturstyrelsen 2013. Natura 2000-basisanalyse 2016-2021. Lille Vildmose, Tofte Skov og Høstemark Skov. Natura 2000-område nr. 17, Habitatområde H18, Fuglebeskyttelsesområde F7. Miljøministeriet.
Naturvårdsverket, 2014. Våtmarksinventeringen uttag av arealdata om ingreppsnivåer per delobjekttyp (Ärende NV-03816-14).
Newbold, T. et al. 2015. Global effects of land use on local terrestrial biodiversity. Nature 520, 45-50.
Nordén, B., Evju, M., Jordal, J.B. 2015. Gamle edelløvtrær – et hotspot-habitat. Sluttrapport under ARKO-prosjektets periode III - NINA Rapport 1168. 91 s.
Nordén, B., Rørstad, P. K., Götmark, F., Magnér, J., Löf, M. 2019. The economy of selective cutting in recent mixed stands during restoration of temperate deciduous forest. Scandinavian Journal of Forest Research 34: 709-717.
Nord-Larsen, T., Johannsen, V. K., Riis-Nielsen, T., Thomsen, I. M., & Jørgensen, B. B. 2019. Skovstatistik 2018: Forest statistics 2018. Institut for Geovidenskab og Naturforvaltning, Department of Geosciences, Frederiksberg 40 pp.
Nord-Larsen, T. Vesterdal, L., Bentsen, N. S., & Larsen, J. B. 2019. Ecosystem carbon stocks and their temporal resilience in a semi-natural beech-dominated forest. Forest Ecology and Management 447: 67–76.
Nugent, K. A., Strachan, I. B., Strack, M., Roulet, N. T., Rochefort, L. 2018. Multi-year net ecosystem carbon balance of a restored peatland reveals a return to a carbon sink. Global Change Biology, 24: 5751-5768.
Ódor, P., Heilmann-Clausen, J., Christensen, M., Aude, E., van Dort, K.W., Piltaver, A., Siller, I., Veerkampd, M. T., Walleyn, R., Standovár, T., van Hees, A. F. M., Kosec, J., Matocec, N., Kraigher, H., & Grebenc T. 2006. Diversity of dead wood inhabiting fungi and bryophytes in semi-natural beech forests in Europe. Biol. Cons. 131: 58–71.
Ojanen, P., Minkkinen, K., Alm, J., Penttilä, T. 2010. Soil–atmosphere CO2, CH4 and N2O fluxes in boreal forestry-drained peatlands. Forest Ecology and Management 260: 411-421.
Ojanen, P., Penttila, T., Tolvanen, A., Hotanene, J-P., Saarimaa, M., Nousiainenb, H., Minkkinena, K. 2018. Long-term effect of fertilization on the greenhouse gas exchange of low-productive peatland forests. Forest Ecology and Management 432: 786–798.
Oksanen, P. O. 2005. Development of palsa mires on the northern European continent in relation to Holocene climatic and environmental changes. Faculty of Science, Department of Biology, University of Oulu, P.O.Box 3000, FIN-90014. University of Oulu, Finland.
Olsen, S. L., Rusch, G. M., Kvakkestad, V., Rønningen, K., Rørstad, P. K. Venter, Z., Nordén, B. 2020. Restaurering av edelløvskog: fortidens skog er fremtidens skog. NINA Temahefte 77. Norsk institutt for naturforskning.
Olvmo, M., Holmer, B., Thorsson, S., Reese, H., Lindberg, F. 2020. Sub-arctic palsa degradation and the role of climatic drivers in the largest coherent palsa mire complex in Sweden (Vissátvuopmi) 1955–2016. Scientific reports 10:8937.
Onarheim, K. 2018. Market and regulatory issues related to Bio-CCUS. Workshop summary. IEA Bioenergy, Task 41.
Ojanen, P., & Minkkinen, K. 2019. The dependence of net soil CO2 emissions on water table depth in boreal peatlands drained for forestry. Mires and Peat, 24, Article 27.
Óskarsson, H. 2009. Hekluskógar – Islands største reetablering af birkeskove. [Hekla Forests – Icelands largest restoration of birch forests] Skoven 01 2009.
Óskarsson, H., Halldórsson, G., Aradóttir, Á. L. 2011. Hekluskógar – large scale restoration of birch woodlands with minimum inputs. In: Restoring the North – Challenges and opportunities. International Restoration Conference, Iceland, October 20-22, 2011. Book of abstracts. Soil Conservation Service of Iceland and Agricultural University of Iceland.
Owona, J. C. 2019. Changes in carbon-stock and soil properties following afforestation in SW Iceland. MsC thesis. Aggriculturel University of Iceland.
Paillet, Y., Bergès, L., Hjältén, J., Ódor, P., Avon, C., et al., 2010. Biodiversity differences between managed and unmanaged forests: meta-analysis of species richness in Europe. Conserv. Biol. 24, 101–112.
Panitz, S., Salzmann, U., Risebrobakken, B., De Schepper, S., and Pound, M. J. 2016. Climate variability and long-term expansion of peatlands in Arctic Norway during the late Pliocene (ODP Site 642, Norwegian Sea), Clim. Past, 12, 1043–1060.
Parish, F., Sirin, A., Charman, D., Joosten, H., Minayeva, T., Silvius, M. and Stringer, L. (Eds.) 2008. Assessment on Peatlands, Biodiversity and Climate Change: Main Report. Global Environment Centre, Kuala Lumpur and Wetlands International, Wageningen.
Payette, S., Delwaide, A., Caccianiga, M., Beauchemin, M. 2004. Accelerated thawing of subarctic peatland permafrost over the last 50 years. Geophysical research letters 31: 1-4.
Peltoniemi, M., Mäkipää, R., Liski, J., Tamminen, P. 2004. Changes in soil carbon with stand age – an evaluation of a modeling method with empirical data. Global Change Biology 10: 2078–2091.
Penttilä, T., Keränen, M., Parviainen, M., Ojanen, P., Haapalehto, T., Minkkinen, K., 2015. Restoration of low-productive, forestry-drained peatlands - impacts on CO2 and CH4 fluxes. LUKE Natural Resources Institute Finland.
Petersen, A. H., Lundhede, T. H., Bruun, H. H., Heilmann-Clausen, J., Thorsen, B. J ., Strange N., & Rahbek, C. 2016. Bevarelse af biodiversiteten i de danske skove. En analyse af den nødvendige indsats, og hvad den betyder for skovens andre samfundsgoder. [Conservation of biodiversity in the Danish forests. An analysis of the effort needed and the significance for other ecosystem services] Center for Macroecology, Evolution and Climate, University of Copenhagen. 110 pp.
Petersen, A. H., Bladt, J., Bruun, H. H., Ejrnæs, R., Heilmann-Clausen, J. & Rahbek C. 2017. Biologiske anbefalinger om udpegning af skov til biodiversitetsformål på statens arealer. Forskningsbaseret rådgivning fra Københavns og Aarhus Universiteter i forbindelse med regeringens Naturpakke. [Biological recommendations on the designation of forest for biodiversity conservation on state owned lands. Research-based advice from the Universities of Aarhus and Copenhagen] Center for Macroecology, Evolution and Climate, University of Copenhagen. 40 pp. (Report in Danish).
Petersen, A. H., Bladt, J., Bruun, H. H., Ejrnæs, R., Heilmann-Clausen, J. & Rahbek C. 2018. Udpegning af skov til biodiversitetsformål på statens arealer: Sammenligning af Naturstyrelsens forslag med anbefalingerne fra KU og AU. [Designation of forest for biodiversity conservation on state owned land: Comparison with the Recommendations from the Universities of Copenhagen and Aarhus.] Center for Macroecology, Evolution and Climate, University of Copenhagen. 8 pp. (Note in Danish).
Petersen, A. H., Johannsen, V. K., Rahbek, C., Beier, C., Bruun, H. H., Heilmann-Clausen, J., Vesterdal, L., Bentsen, N. S., Gundersen, P., Nord-Larsen, T. 2020. Notat on klimaeffekt af urørt skov [Note on the climate effect of unmanaged forest]. Sagsnotat. 4 pp. (Note in Danish).
Purvis, A., Newbold, T., De Palma, A., Contu, S., et al. 2018. Modelling and Projecting the Response of Local Terrestrial Biodiversity Worldwide to Land Use and Related Pressures: The PREDICTS Project. Advances in Ecological Research 58.
Ramsar Information Sheet 2013. Information Sheet on Ramsar Wetlands (RIS) – 2009-2012. Lille Vildmose, Denmark. Ramsar Convention on Wetlands.
Ramsar 2015. Resolution XII.11. Peatlands, climate change and wise use: Implications for the Ramsar Convention. 12th Meeting of the Conference of the Parties to the Convention on Wetlands. Ponte del Este, Uruguay.
Ramsar 2018. Resolution XIII.12 Guidance on identifying peatlands as Wetlands of International Importance (Ramsar Sites) for global climate change regulation as an additional argument to existing Ramsar criteria. 13th Meeting of the Conference of the Contracting Parties to the Ramsar Convention on Wetlands “Wetlands for a Sustainable Urban Future” Dubai, United Arab Emirates.
Romppanen, S. 2020. The EU Effort Sharing and LULUCF Regulations: The Complementary yet Crucial Components of the EU’s Climate Policy Beyond 2030 (May 1, 2019). Research Handbook of European Environmental Law, edited by Marjan Peeters and Mariolina Eliantonio, Edward Elgar Publishing Ltd.
Rosenvald, R. & Lohmus, A. 2008. For what, when, and where is green-tree retention better than clear-cutting? A review of the biodiversity aspects. Forest Ecology and Management 255: 1–15.
Rudolphi, J. & Gustafsson, L. 2011. Forests regenerating after clear-cutting function as habitat for bryophyte and lichen species of conservation concern. PLoS One 6, e18639.
Rusch, G. M. 2012. Climate and ecosystem services. The potential of Norwegian ecosystems for cli-mate mitigation and adaptation. NINA Report 791. Norwegian Institute for Nature Research.
Råghall, J. 2019. Fågelinventering av Tysjöarna, Krokom Kommun, Jämtland.
Saarimaa, M., Aapala, K., Tuominen, S., Karhu, J., Parkkari, M. & Tolvanen, A. 2019. Predicting hotspots for threatened plant species in boreal peatlands. Biodiversity and Conservation (2019) 28:1173–1204.
Sathre, R., O'Connor, J., 2010. Meta-analysis of greenhouse gas displacement factors of wood product substitution. Environ. Sci. Policy 13, 104-114.
Scholes, R. J. & Biggs, R. 2005. A biodiversity intactness index. Nature 434, 45-49.
Schuur, E., McGuire, A., Schädel, C. et al. 2015. Climate change and the permafrost carbon feedback. Nature 520: 171–179.
Searchinger, T. D., Beringer, T., Holtsmark, B., Kammen, D. M., Lambin, E. F., Lucht, W., Raven, P., van Ypersele, J. P., 2018. Europe’s renewable energy directive poised to harm global forests. Nat. Commun. 9. 3741.
Sigurðsson, S. 1977. Birki á Íslandi (Birch in Iceland), in Skógarmál, Guðmundsson, H., Ed., Edda Print, Reykjavík.
Sirin, A. A., Medvedeva, M. A., Makarov, D. A., Maslov, A. A., Joosten, H. 2020. Multispectral satellite based monitoring of land cover change and associated fire reduction after large-scale peatland rewetting following the 2010 peat fires in Moscow Region (Russia). Ecological Engineering 158.
Snorrason, A., Jónsson, T. H. & Eggertsson, Ó. 2019. Aboveground woody biomass of natural birch woodland in Iceland – Comparison of two inventories 1987-1988 and 2005-2011 Icel. Agric. Sci. 32: 21-29.
Snorrason, A., Sigurdsson, B. D., Guðbergsson, G., Svavarsdóttir, K., Jónsson, Þ. H. 2002. Carbon sequestration in forest plantations in Iceland. Icelandic Agricultural Sciences, 15: 81-93.
Snorrason A, Traustason, B., Kjartansson, BÞ., Heiðarsson, L., Ísleifsson, R., Eggertsson, Ó. 2016. Náttúrulegt birki á Íslandi – ný úttekt á útbreiðslu þess og ástandi. [Natural birch woodland in Iceland – a new assessment on distribution and state]. Náttúrufræðingurinn 86: 97-111.
Spake, R., Ezard, T. H. G., Martin, P.A., Newton, A. C. & Doncaster, C. P. 2015. A meta-analysis of functional group responses to forest recovery outside of the tropics. Conservation Biology 29: 1695–1703.
Statistiska centralbyrån (SCB) 2013. Torv 2012 Produktion, anvanding, miljöeffekter. Statistiske meddelanden MI 25 SM 1303.
Sterkenburg, E., Bahr, A., Brandström Durling, M., Clemmensen, K. E., Lindahl, B. D. 2015. Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol. 207: 1145–1158.
Sterkenburg, E., Clemmensen, K. E., Lindahl, B. D., Dahlberg, A. 2019. The significance of retention trees for survival of ectomycorrhizal fungi in clear-cut Scots pine forests. J. Appl. Ecol. 56:1367–1378.
Taeroe, A., Fayez Mustapha, W., Stupak, I., Raulund-Rasmussen, K. 2017. Do forests best mitigate CO2 emissions to the atmosphere by setting them aside for maximization of carbon storage or by management for fossil fuel substitution? J. Environ. Manage. 197, 117–129.
Tarnocai, C., Canadell, J. G., Schuur, E. A. G., Kuhry, P., Mazhitova, G., Zimov, S. 2009. Soil organic carbon pools in the northern circumpolar permafrost region, Global Biogeochem. Cycles, 23, GB2023.
Tenning, L. 2015. Life to ad(d)mire. Restoration of wetlands and mires in seven counties. The County Administrative Board of Jämtland.
The Danish Nature Agency. 2018. Endelig udpegning af skov til biodiversitetsformål [Final designation of forest for biodiversity purposes]. The Danish Nature Agency. 38 pp. [Report in Danish).
Tolvannen, A., Pavieinen, M. 2015. Quantification and valuation of ecosystem services to optimize sustainable re-use for low-productive drained peatlands. Raisa Mäkipää and Tuire Kilponen (eds.) Towards a New Era of Forest Science in the Boreal Region. Conference, Rovaniemi, Finland.
Tolvanen, A., Tarvainen1, O. & Laine, A. M. 2020a. Soil and water nutrients in stem-only and whole-tree harvest treatments in restored boreal peatlands. Restoration Ecology 1-8.
Tolvanen, A., Saarimaa, M., Tuominen, S., Aapala, K. 2020b. Is 15% restoration sufficient to safeguard the habitats of boreal red-listed mire plant species? Global Ecology and Conservation 23: e01160.
Tomter, S. M., Dalen, L.S. (eds). 2018. Bærekraftig skogbruk i Norge. Norsk institutt for bioøkonomi.
Trbojević, N. 2016. The Impact of Settlement on Woodland Resources in Viking Age Iceland. PhD thesis. University of Iceland. Reykjavík. 223 pp.
Ťupek B, Launiainen S, Peltoniemi M, Sievänen R, Perttunen J, Kulmala L, Penttilä T, Lindroos A-J, Hashimoto S, Lehtonen A. 2019. Evaluating CENTURY and Yasso soil carbon models for CO2 emissions and organic carbon stocks of boreal forest soil with Bayesian multi-model inference. European Journal of Soil Science, 70: 847-858.
UNEP 2019. United Nations Decade on Ecosystem Restoration. Resolution adopted by the General Assembly on 1 March 2019. https://undocs.org/A/RES/73/284
Wardle, D. A., Zackrisson, O., Hörnberg, G., Gallet, C. 1997. Influence of island area on ecosystem properties. Science 277: 1296–1299.
Wardle, D. A., Walker, L. R. Bardgett, R. D. 2004. Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305: 509–513.
Wardle, D. A., Jonsson, M., Bansal, S., Bardgett, R. D., Gundale, M. J., Metcalfe, D. B. 2012. Linking vegetation change, carbon sequestration and biodiversity: insights from island ecosystems in a long-term natural experiment. Journal of Ecology 100:16–30.
Wichtmann, W., Schröder, C., Joosten, H. 2016. Paludiculture - productive use of wet peatlands. Climate protection - biodiversity - regional economic benefits. Schweizerbart, Science Publishers.
World Energy Council, 2013. World Energy Resources: Peat. World Energy Resources 2013 Survey.
Wöll, C. 2008. Treeline of mountain birch (Betula pubescens Ehrh.) in Iceland and its relationship to temperature. Diploma thesis in Forest Botany. Technical University Dresden. 125 pp.
Zackrisson, O. 1977. Influence of forest fires on the north Swedish boreal forest. Oikos 29: 22–32.
About this publication
Synergy in conservation of biodiversity and climate change mitigation
– Nordic peatlands and forests
Lars Dinesen, Anders Højgård Petersen and Carsten Rahbek
ISBN 978-92-893-6951-0 (PDF)
ISBN 978-92-893-6952-7 (ONLINE)
http://dx.doi.org/10.6027/temanord2021-510
TemaNord 2021:510
ISSN 0908-6692
Cover photo: Lars Petersson
© Nordic Council of Ministers 2021
Disclaimer
This publication was funded by the Nordic Council of Ministers. However, the content does not necessarily reflect the Nordic Council of Ministers’ views, opinions, attitudes or recommendations.
Rights and permissions
This work is made available under the Creative Commons Attribution 4.0 International license (CC BY 4.0) https://creativecommons.org/licenses/by/4.0.
Translations: If you translate this work, please include the following disclaimer: This translation was not produced by the Nordic Council of Ministers and should not be construed as official. The Nordic Council of Ministers cannot be held responsible for the translation or any errors in it.
Adaptations: If you adapt this work, please include the following disclaimer along with the attribution: This is an adaptation of an original work by the Nordic Council of Ministers. Responsibility for the views and opinions expressed in the adaptation rests solely with its author(s). The views and opinions in this adaptation have not been approved by the Nordic Council of Ministers.
Third-party content: The Nordic Council of Ministers does not necessarily own every single part of this work. The Nordic Council of Ministers cannot, therefore, guarantee that the reuse of third-party content does not infringe the copyright of the third party. If you wish to reuse any third-party content, you bear the risks associated with any such rights violations. You are responsible for determining whether there is a need to obtain permission for the use of third-party content, and if so, for obtaining the relevant permission from the copyright holder. Examples of third-party content may include, but are not limited to, tables, figures or images.
Photo rights (further permission required for reuse):
Any queries regarding rights and licences should be addressed to:
Nordic Council of Ministers/Publication Unit
Ved Stranden 18
DK-1061 Copenhagen
Denmark
pub@norden.org
Nordic co-operation
Nordic co-operation is one of the world’s most extensive forms of regional collaboration, involving Denmark, Finland, Iceland, Norway, Sweden, and the Faroe Islands, Greenland and Åland.
Nordic co-operation has firm traditions in politics, economics and culture and plays an important role in European and international forums. The Nordic community strives for a strong Nordic Region in a strong Europe.
Nordic co-operation promotes regional interests and values in a global world. The values shared by the Nordic countries help make the region one of the most innovative and competitive in the world.
The Nordic Council of Ministers
Nordens Hus
Ved Stranden 18
DK-1061 Copenhagen
pub@norden.org
Read more Nordic publications on www.norden.org/publications